Population pharmacokinetics of nefopam in elderly, with or without renal impairment, and its link to treatment response
- PMID: 24252055
- PMCID: PMC4093928
- DOI: 10.1111/bcp.12291
Population pharmacokinetics of nefopam in elderly, with or without renal impairment, and its link to treatment response
Abstract
Aims: Nefopam is a nonmorphinic central analgesic, for which no recommendation exists concerning adaptation of regimen in aged patients with or without renal impairment. The objective was to describe the pharmacology of nefopam in aged patients to obtain guidelines for practical use.
Methods: Elderly patients (n = 48), 65-99 years old, with severe or moderate renal impairment or with normal renal function, were recruited. Nefopam (20 mg) was administered as a 30 min infusion postoperatively. Simultaneously, a 1 min intravenous infusion of iohexol was performed, in order to calculate the glomerular filtration rate. Blood samples were drawn to determine nefopam, desmethyl-nefopam and iohexol plasma concentrations. Nefopam and desmethyl-nefopam concentrations were analysed using a nonlinear mixed-effects modelling approach with Monolix version 4.1.3. The association between pharmacokinetic parameters and treatment response was assessed using logistic regression.
Results: A two-compartment open model was selected to describe the pharmacokinetics of nefopam. The typical population estimates (between-subject variability) for clearance, volume of distribution, intercompartmental clearance and peripheral volume were, respectively, 17.3 l h(-1) (53.2%), 114 l (121%), 80.7 l h(-1) (79%) and 208 l (63.6%). Morphine requirement was related to exposure of nefopam. Tachycardia and postoperative nausea and vomiting were best associated with maximal concentration and the rate of increase in nefopam plasma concentration.
Conclusions: We identified the nefopam pharmacokinetic predictors for morphine requirement and side-effects, such as tachycardia and postoperative nausea and vomiting. In order to maintain morphine sparing and decrease side-effects following a single dose of nefopam (20 mg), simulations suggest an infusion time of >45 min in elderly patients with or without renal impairment.
Keywords: analgesia; elderly; logistic regression; nefopam; population pharmacokinetics; renal impairment.
© 2013 The British Pharmacological Society.
Figures




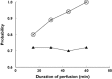
 , Cmaxnef <389 μg l−1;
, Cmaxnef <389 μg l−1;  , RCmaxnef <764 μg l−1;
, RCmaxnef <764 μg l−1;  , AUCnef0→∞ >950 μg h l−1
, AUCnef0→∞ >950 μg h l−1References
-
- Aubrun F, Marmion F. The elderly patient and postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007;21:109–127. - PubMed
-
- Zyczkowska J, Szczerbińska K, Jantzi MR, Hirdes JP. Pain among the oldest old in community and institutional settings. Pain. 2007;129:167–176. - PubMed
-
- Abdulla A, Bone M, Adams N, Elliott AM, Jones D, Knaggs R, Martin D, Sampson EL, Schofield P. Evidence-based clinical practice guidelines on management of pain in older people. Age Ageing. 2013;42:151–153. - PubMed
-
- Aubrun F. Management of postoperative analgesia in elderly patients. Reg Anesth Pain Med. 2005;30:363–379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

