Differential expression of neuregulin-1 isoforms and downregulation of erbin are associated with Erb B2 receptor activation in diabetic peripheral neuropathy
- PMID: 24252174
- PMCID: PMC3893607
- DOI: 10.1186/2051-5960-1-39
Differential expression of neuregulin-1 isoforms and downregulation of erbin are associated with Erb B2 receptor activation in diabetic peripheral neuropathy
Abstract
Background: Aberrant neuron/glia interactions can contribute to a variety of neurodegenerative diseases and we have previously demonstrated that enhanced activation of Erb B2, which is a member of the epidermal growth factor receptor (EGFR) family, can contribute to the development of diabetic peripheral neuropathy (DPN). In peripheral nerves, Erb B receptors are activated by various members of the neuregulin-1 (NRG1) family including NRG1 Type I, NRG1 Type II and NRG1 Type III to regulate Schwann cell (SC) growth, migration, differentiation and dedifferentiation. Alternatively, Erb B2 activity can be negatively regulated by association with the Erb B2-interacting protein, erbin. Since the effect of diabetes on the expression of NRG1 isoforms and erbin in peripheral nerve are unknown, the current study determined whether changes in NRG1 isoforms and erbin may be associated with altered Erb B2 signaling in DPN.
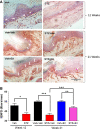
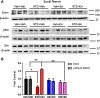
Results: Swiss Webster mice were rendered diabetic with streptozotocin (STZ) and after 12 weeks of diabetes, treated with erlotinib, an inhibitor of Erb B2 activation. Inhibition of Erb B2 signaling partially reversed several pathophysiologic aspects of DPN including a pronounced sensory hypoalgesia, nerve conduction velocity deficits and the decrease in epidermal nerve fiber innervation. We also observed a decrease of NRG1 Type III but an increase of NRG1 Type I level in diabetic sural nerves at early stage of diabetes. With disease progression, we detected reduced erbin expression and enhanced MAPK pathway activity in diabetic mice. Inhibition of Erb B2 receptor suppressed MAPK pathway activity in treated-diabetic sural nerves.
Conclusions: These results support that hyperglycemia may impair NRG1/Erb B2 signaling by disrupting the balance between NRG1 isoforms, decreasing the expression of erbin and correspondingly activating the MAPK pathway. Together, imbalanced NRG1 isoforms and downregulated erbin may contribute to the dysregulation of Erb B2 signaling in the development of DPN.
Figures






Similar articles
-
Caveolin-1 and altered neuregulin signaling contribute to the pathophysiological progression of diabetic peripheral neuropathy.Diabetes. 2009 Nov;58(11):2677-86. doi: 10.2337/db09-0594. Epub 2009 Aug 12. Diabetes. 2009. PMID: 19675140 Free PMC article.
-
Erbin regulates NRG1 signaling and myelination.Proc Natl Acad Sci U S A. 2009 Jun 9;106(23):9477-82. doi: 10.1073/pnas.0901844106. Epub 2009 May 20. Proc Natl Acad Sci U S A. 2009. PMID: 19458253 Free PMC article.
-
Erbin is required for myelination in regenerated axons after injury.J Neurosci. 2012 Oct 24;32(43):15169-80. doi: 10.1523/JNEUROSCI.2466-12.2012. J Neurosci. 2012. PMID: 23100438 Free PMC article.
-
The neuregulin-I/ErbB signaling system in development and disease.Adv Anat Embryol Cell Biol. 2007;190:1-65. Adv Anat Embryol Cell Biol. 2007. PMID: 17432114 Review.
-
Altered neurotrophism in diabetic neuropathy: spelunking the caves of peripheral nerve.J Pharmacol Exp Ther. 2005 May;313(2):485-91. doi: 10.1124/jpet.104.079921. Epub 2004 Dec 17. J Pharmacol Exp Ther. 2005. PMID: 15608075 Review.
Cited by
-
Novel Molecular Networks and Regulatory MicroRNAs in Type 2 Diabetes Mellitus: Multiomics Integration and Interactomics Study.JMIR Bioinform Biotechnol. 2022 Feb 23;3(1):e32437. doi: 10.2196/32437. JMIR Bioinform Biotechnol. 2022. PMID: 38935970 Free PMC article.
-
Overexpression of Neuregulin-1 Type III Has Impact on Visual Function in Mice.Int J Mol Sci. 2022 Apr 19;23(9):4489. doi: 10.3390/ijms23094489. Int J Mol Sci. 2022. PMID: 35562880 Free PMC article.
-
Design of Mixed Medicinal Plants, Rich in Polyphenols, Vitamins B, and Palmitoylethanolamide-Based Supplement to Help Reduce Nerve Pain: A Preclinical Study.Int J Mol Sci. 2024 Apr 27;25(9):4790. doi: 10.3390/ijms25094790. Int J Mol Sci. 2024. PMID: 38732008 Free PMC article.
-
Effects of Nutraceutical Compositions Containing Rhizoma Gastrodiae or Lipoic Acid in an In Vitro Induced Neuropathic Pain Model.Int J Mol Sci. 2024 Feb 17;25(4):2376. doi: 10.3390/ijms25042376. Int J Mol Sci. 2024. PMID: 38397054 Free PMC article.
-
Neuregulin 1 improves complex 2-mediated mitochondrial respiration in skeletal muscle of healthy and diabetic mice.Sci Rep. 2017 May 11;7(1):1742. doi: 10.1038/s41598-017-02029-z. Sci Rep. 2017. PMID: 28496106 Free PMC article.
References
-
- Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve. 2007;1:144–166. - PubMed
-
- Sinnreich M, Taylor BV, Dyck PJ. Diabetic neuropathies. Classification, clinical features, and pathophysiological basis. Neurologist. 2005;1:63–79. - PubMed
-
- Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1:931–940. - PubMed
-
- Calcutt NA, Jolivalt CG, Fernyhough P. Growth factors as therapeutics for diabetic neuropathy. Curr Drug Targets. 2008;1:47–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

