The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D)
- PMID: 24252196
- PMCID: PMC3776212
- DOI: 10.1186/2051-5960-1-4
The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D)
Abstract
Background: To investigate the association of DNA nucleotide excision repair (NER) defects with neurological degeneration, cachexia and cancer, we performed autopsies on 4 adult xeroderma pigmentosum (XP) patients with different clinical features and defects in NER complementation groups XP-A, XP-C or XP-D.
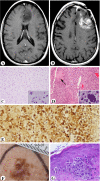
Results: The XP-A (XP12BE) and XP-D (XP18BE) patients exhibited progressive neurological deterioration with sensorineural hearing loss. The clinical spectrum encompassed severe cachexia in the XP-A (XP12BE) patient, numerous skin cancers in the XP-A and two XP-C (XP24BE and XP1BE) patients and only few skin cancers in the XP-D patient. Two XP-C patients developed internal neoplasms including glioblastoma in XP24BE and uterine adenocarcinoma in XP1BE. At autopsy, the brains of the 44 yr XP-A and the 45 yr XP-D patients were profoundly atrophic and characterized microscopically by diffuse neuronal loss, myelin pallor and gliosis. Unlike the XP-A patient, the XP-D patient had a thickened calvarium, and the brain showed vacuolization of the neuropil in the cerebrum, cerebellum and brainstem, and patchy Purkinje cell loss. Axonal neuropathy and chronic denervation atrophy of the skeletal muscles were observed in the XP-A patient, but not in the XP-D patient.
Conclusions: These clinical manifestations and autopsy findings indicate advanced involvement of the central and peripheral nervous system. Despite similar defects in DNA repair, different clinicopathological phenotypes are seen in the four cases, and therefore distinct patterns of neurodegeneration characterize XP-D, XP-A and XP-C patients.
Figures


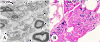





Similar articles
-
Auditory analysis of xeroderma pigmentosum 1971-2012: hearing function, sun sensitivity and DNA repair predict neurological degeneration.Brain. 2013 Jan;136(Pt 1):194-208. doi: 10.1093/brain/aws317. Brain. 2013. PMID: 23365097 Free PMC article.
-
Differences in peripheral neuropathy in xeroderma pigmentosum complementation groups A and D as evaluated by nerve conduction studies.BMC Neurol. 2021 Oct 9;21(1):393. doi: 10.1186/s12883-021-02414-2. BMC Neurol. 2021. PMID: 34627174 Free PMC article.
-
Shining a light on xeroderma pigmentosum.J Invest Dermatol. 2012 Mar;132(3 Pt 2):785-96. doi: 10.1038/jid.2011.426. Epub 2012 Jan 5. J Invest Dermatol. 2012. PMID: 22217736 Free PMC article. Review.
-
[Xeroderma pigmentosum].Nihon Rinsho. 2000 Jul;58(7):1496-500. Nihon Rinsho. 2000. PMID: 10921330 Review. Japanese.
-
A novel mutation in the XPA gene associated with unusually mild clinical features in a patient who developed a spindle cell melanoma.Br J Dermatol. 2006 Jul;155(1):81-8. doi: 10.1111/j.1365-2133.2006.07272.x. Br J Dermatol. 2006. PMID: 16792756 Review.
Cited by
-
Living with xeroderma pigmentosum: comprehensive photoprotection for highly photosensitive patients.Photodermatol Photoimmunol Photomed. 2014 Apr-Jun;30(2-3):146-52. doi: 10.1111/phpp.12108. Epub 2014 Feb 19. Photodermatol Photoimmunol Photomed. 2014. PMID: 24417420 Free PMC article. Review.
-
Xeroderma Pigmentosum: A Model for Human Premature Aging.J Invest Dermatol. 2021 Apr;141(4S):976-984. doi: 10.1016/j.jid.2020.11.012. Epub 2021 Jan 9. J Invest Dermatol. 2021. PMID: 33436302 Free PMC article. Review.
-
POLD3 haploinsufficiency is linked to non-syndromic sensorineural adult-onset progressive hearing and balance impairments.Eur J Hum Genet. 2025 Jan;33(1):121-130. doi: 10.1038/s41431-024-01715-7. Epub 2024 Oct 16. Eur J Hum Genet. 2025. PMID: 39414923
-
Skin Signals: Exploring the Intersection of Cancer Predisposition Syndromes and Dermatological Manifestations.Int J Mol Sci. 2025 Jun 26;26(13):6140. doi: 10.3390/ijms26136140. Int J Mol Sci. 2025. PMID: 40649916 Free PMC article. Review.
-
Case Report: Identification of a Heterozygous XPA c.553C>T Mutation Causing Neurological Impairment in a Case of Xeroderma Pigmentosum Complementation Group A.Front Genet. 2021 Aug 16;12:717361. doi: 10.3389/fgene.2021.717361. eCollection 2021. Front Genet. 2021. PMID: 34484303 Free PMC article.
References
-
- Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, Oh KS, Imoto K, Inui H, Moriwaki S, Emmert S, Pike KM, Raziuddin A, Plona TM, DiGiovanna JJ, Tucker MA, Kraemer KH. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168–176. doi: 10.1136/jmg.2010.083022. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

