The resistance mechanisms of proteasome inhibitor bortezomib
- PMID: 24252210
- PMCID: PMC4177604
- DOI: 10.1186/2050-7771-1-13
The resistance mechanisms of proteasome inhibitor bortezomib
Abstract
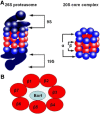
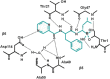
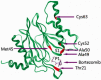
The proteasome inhibitor, bortezomib, a boronic dipeptide which reversibly inhibit the chymotrypsin-like activity at the β5-subunit of proteasome (PSMB5), has marked efficacy against multiple myeloma and several non-Hodgkin's lymphoma subtypes, and has a potential therapeutic role against other malignancy diseases. However, intrinsic and acquired resistance to bortezomib may limit its efficacy. In this article, we discuss recent advances in the molecular understanding of bortezomib resistance. Resistance mechanisms discussed include mutations of PSMB5 and the up-regulation of proteasome subunits, alterations of gene and protein expression in stress response, cell survival and antiapoptotic pathways, and multidrug resistance.
Figures

Similar articles
-
Molecular basis of bortezomib resistance: proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein.Blood. 2008 Sep 15;112(6):2489-99. doi: 10.1182/blood-2007-08-104950. Epub 2008 Jun 18. Blood. 2008. PMID: 18565852
-
Interferon-γ-induced upregulation of immunoproteasome subunit assembly overcomes bortezomib resistance in human hematological cell lines.J Hematol Oncol. 2014 Jan 13;7:7. doi: 10.1186/1756-8722-7-7. J Hematol Oncol. 2014. PMID: 24418325 Free PMC article.
-
Proteasome-based mechanisms of intrinsic and acquired bortezomib resistance in non-small cell lung cancer.Biochem Pharmacol. 2012 Jan 15;83(2):207-17. doi: 10.1016/j.bcp.2011.10.009. Epub 2011 Oct 18. Biochem Pharmacol. 2012. PMID: 22027222
-
The emerging role of bortezomib in the treatment of indolent non-Hodgkin's and mantle cell lymphomas.Curr Treat Options Oncol. 2004 Aug;5(4):269-81. doi: 10.1007/s11864-004-0018-2. Curr Treat Options Oncol. 2004. PMID: 15233904 Review.
-
Proteasome inhibitors in the treatment of B-cell malignancies.Clin Lymphoma. 2002 Jun;3(1):49-55. doi: 10.3816/clm.2002.n.011. Clin Lymphoma. 2002. PMID: 12141956 Review.
Cited by
-
The NEDD4-1 E3 ubiquitin ligase: A potential molecular target for bortezomib sensitivity in multiple myeloma.Int J Cancer. 2020 Apr 1;146(7):1963-1978. doi: 10.1002/ijc.32615. Epub 2019 Aug 24. Int J Cancer. 2020. PMID: 31390487 Free PMC article.
-
De novo macrocyclic peptides that specifically modulate Lys48-linked ubiquitin chains.Nat Chem. 2019 Jul;11(7):644-652. doi: 10.1038/s41557-019-0278-x. Epub 2019 Jun 10. Nat Chem. 2019. PMID: 31182821 Free PMC article.
-
Unbiased compound-protein interface mapping and prediction of chemoresistance loci through forward genetics in haploid stem cells.Oncotarget. 2018 Jan 23;9(11):9838-9851. doi: 10.18632/oncotarget.24305. eCollection 2018 Feb 9. Oncotarget. 2018. PMID: 29515774 Free PMC article.
-
Different Strategies to Overcome Resistance to Proteasome Inhibitors-A Summary 20 Years after Their Introduction.Int J Mol Sci. 2024 Aug 16;25(16):8949. doi: 10.3390/ijms25168949. Int J Mol Sci. 2024. PMID: 39201634 Free PMC article. Review.
-
Bortezomib sensitivity is tissue dependent and high expression of the 20S proteasome precludes good response in malignant pleural mesothelioma.Cancer Manag Res. 2019 Sep 24;11:8711-8720. doi: 10.2147/CMAR.S194337. eCollection 2019. Cancer Manag Res. 2019. PMID: 31576173 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

