Epilepsy: neuroinflammation, neurodegeneration, and APOE genotype
- PMID: 24252240
- PMCID: PMC3893449
- DOI: 10.1186/2051-5960-1-41
Epilepsy: neuroinflammation, neurodegeneration, and APOE genotype
Abstract
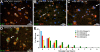
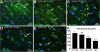
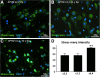
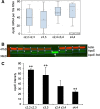
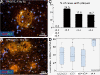
Background: Precocious development of Alzheimer-type neuropathological changes in epilepsy patients, especially in APOE ϵ4,4 carriers is well known, but not the ways in which other APOE allelic combinations influence this outcome. Frozen and paraffin-embedded tissue samples resected from superior temporal lobes of 92 patients undergoing temporal lobectomies as a treatment for medication-resistant temporal lobe epilepsy were used in this study. To determine if epilepsy-related changes reflect those in another neurological condition, analogous tissue samples harvested from 10 autopsy-verified Alzheimer brains, and from 10 neurologically and neuropathologically normal control patients were analyzed using immunofluorescence histochemistry, western immunoblot, and real-time PCR to determine genotype effects on neuronal number and size, neuronal and glial expressions of amyloid β (Aβ) precursor protein (βAPP), Aβ, apolipoprotein E (ApoE), S100B, interleukin-1α and β, and α and β secretases; and on markers of neuronal stress, including DNA/RNA damage and caspase 3 expression.
Results: Allelic combinations of APOE influenced each epilepsy-related neuronal and glial response measured as well as neuropathological change. APOE ϵ3,3 conferred greatest neuronal resilience denoted as greatest production of the acute phase proteins and low neuronal stress as assessed by DNA/RNA damage and caspase-3 expression. Among patients having an APOE ϵ2 allele, none had Aβ plaques; their neuronal sizes, like those with APOE ϵ3,3 genotype were larger than those with other genotypes. APOE ϵ4,4 conferred the weakest neuronal resilience in epilepsy as well as in Alzheimer patients, but there were no APOE genotype-dependent differences in these parameters in neurologically normal patients.
Conclusions: Our findings provide evidence that the strength of the neuronal stress response is more related to patient APOE genotype than to either the etiology of the stress or to the age of the patient, suggesting that APOE genotyping may be a useful tool in treatment decisions.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

