Myelin alters the inflammatory phenotype of macrophages by activating PPARs
- PMID: 24252308
- PMCID: PMC3893408
- DOI: 10.1186/2051-5960-1-43
Myelin alters the inflammatory phenotype of macrophages by activating PPARs
Abstract
Background: Foamy macrophages, containing myelin degradation products, are abundantly found in active multiple sclerosis (MS) lesions. Recent studies have described an altered phenotype of macrophages after myelin internalization. However, mechanisms by which myelin affects the phenotype of macrophages and how this phenotype influences lesion progression remain unclear.
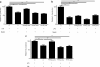
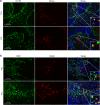
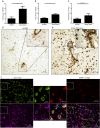
Results: We demonstrate that myelin as well as phosphatidylserine (PS), a phospholipid found in myelin, reduce nitric oxide production by macrophages through activation of peroxisome proliferator-activated receptor β/δ (PPARβ/δ). Furthermore, uptake of PS by macrophages, after intravenous injection of PS-containing liposomes (PSLs), suppresses the production of inflammatory mediators and ameliorates experimental autoimmune encephalomyelitis (EAE), an animal model of MS. The protective effect of PSLs in EAE animals is associated with a reduced immune cell infiltration into the central nervous system and decreased splenic cognate antigen specific proliferation. Interestingly, PPARβ/δ is activated in foamy macrophages in active MS lesions, indicating that myelin also activates PPARβ/δ in macrophages in the human brain.
Conclusion: Our data show that myelin modulates the phenotype of macrophages by PPAR activation, which may subsequently dampen MS lesion progression. Moreover, our results suggest that myelin-derived PS mediates PPARβ/δ activation in macrophages after myelin uptake. The immunoregulatory impact of naturally-occurring myelin lipids may hold promise for future MS therapeutics.
Figures


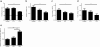
References
-
- Miller E, Mrowicka M, Zolynski K, Kedziora J. [Oxidative stress in multiple sclerosis] PolMerkur Lekarski. 2009;1:499–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

