Integrin α1 subunit is up-regulated in colorectal cancer
- PMID: 24252313
- PMCID: PMC4177608
- DOI: 10.1186/2050-7771-1-16
Integrin α1 subunit is up-regulated in colorectal cancer
Abstract
Background: Colorectal cancer remains one of the leading causes of death from cancer in industrialized countries. Integrins are a family of heterodimeric glycoproteins involved in bidirectional cell signaling and participate in the regulation of cell shape, adhesion, migration, differentiation, gene transcription, survival and proliferation. The α1 subunit is known to be involved in RAS/ERK proliferative pathway activation and plays an important role in mammary carcinoma cell proliferation and migration. In the small intestine, α1 is present in the crypt proliferative compartment and absent in the villus, but nothing is known about its expression in the colon mucosa, or in colorectal cancer.
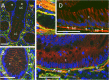
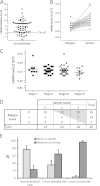
Results: In the present study, we demonstrated that in the colon mucosa, α1 is present in the basolateral domain of the proliferative cells of the crypt, and in the surrounding myofibroblasts. We found higher levels of α1 mRNA in 86% of tumours compared to their corresponding matched margin tissues. Immunohistochemical analysis showed that α1 staining was moderate to high in 65% of tumour cells and 97% of the reactive cells surrounding the tumour cells vs 23% of normal epithelial cells.
Conclusion: Our findings suggest an active role for the α1β1 integrin in colorectal cancer progression.
Figures

Similar articles
-
Involvement of the Integrin α1β1 in the Progression of Colorectal Cancer.Cancers (Basel). 2017 Jul 26;9(8):96. doi: 10.3390/cancers9080096. Cancers (Basel). 2017. PMID: 28933766 Free PMC article.
-
Integrin α1 promotes tumorigenicity and progressive capacity of colorectal cancer.Int J Biol Sci. 2020 Jan 16;16(5):815-826. doi: 10.7150/ijbs.37275. eCollection 2020. Int J Biol Sci. 2020. PMID: 32071551 Free PMC article.
-
Integrin α1β1 expression is controlled by c-MYC in colorectal cancer cells.Oncogene. 2016 Mar 31;35(13):1671-8. doi: 10.1038/onc.2015.231. Epub 2015 Jun 22. Oncogene. 2016. PMID: 26096932 Free PMC article.
-
Colorectal cancer and the integrin family of cell adhesion receptors: current status and future directions.Eur J Cancer. 1994;30A(14):2166-70. doi: 10.1016/0959-8049(94)00473-i. Eur J Cancer. 1994. PMID: 7857718 Review.
-
The gastrointestinal tract stem cell niche.Stem Cell Rev. 2006;2(3):203-12. doi: 10.1007/s12015-006-0048-1. Stem Cell Rev. 2006. PMID: 17625256 Review.
Cited by
-
Reciprocal Interplay Between Fibrillar Collagens and Collagen-Binding Integrins: Implications in Cancer Progression and Metastasis.Front Oncol. 2020 Aug 18;10:1488. doi: 10.3389/fonc.2020.01488. eCollection 2020. Front Oncol. 2020. PMID: 33014790 Free PMC article. Review.
-
MYC Regulates α6 Integrin Subunit Expression and Splicing Under Its Pro-Proliferative ITGA6A Form in Colorectal Cancer Cells.Cancers (Basel). 2018 Feb 3;10(2):42. doi: 10.3390/cancers10020042. Cancers (Basel). 2018. PMID: 29401653 Free PMC article.
-
Therapeutic aspects of c-MYC signaling in inflammatory and cancerous colonic diseases.World J Gastroenterol. 2016 Sep 21;22(35):7938-50. doi: 10.3748/wjg.v22.i35.7938. World J Gastroenterol. 2016. PMID: 27672289 Free PMC article. Review.
-
A biocatalytic peptidobiosensing molecular bridge for detecting osteosarcoma marker protein.Front Chem. 2023 Jan 12;10:1112111. doi: 10.3389/fchem.2022.1112111. eCollection 2022. Front Chem. 2023. PMID: 36712990 Free PMC article.
-
Derivate Isocorydine (d-ICD) Suppresses Migration and Invasion of Hepatocellular Carcinoma Cell by Downregulating ITGA1 Expression.Int J Mol Sci. 2017 Feb 27;18(3):514. doi: 10.3390/ijms18030514. Int J Mol Sci. 2017. PMID: 28264467 Free PMC article.
References
-
- Aplin AE, Juliano RL. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J Cell Sci. 1999;112(Pt 5):695–706. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

