Friedreich ataxia: metal dysmetabolism in dorsal root ganglia
- PMID: 24252376
- PMCID: PMC3893523
- DOI: 10.1186/2051-5960-1-26
Friedreich ataxia: metal dysmetabolism in dorsal root ganglia
Abstract
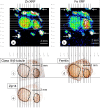
Background: Friedreich ataxia (FA) causes distinctive lesions of dorsal root ganglia (DRG), including neuronal atrophy, satellite cell hyperplasia, and absorption of dying nerve cells into residual nodules. Two mechanisms may be involved: hypoplasia of DRG neurons from birth and superimposed iron (Fe)- and zinc (Zn)-mediated oxidative injury. This report presents a systematic analysis of DRG in 7 FA patients and 13 normal controls by X-ray fluorescence (XRF) of polyethylene glycol-embedded DRG; double-label confocal immunofluorescence microscopy of Zn- and Fe-related proteins; and immunohistochemistry of frataxin and the mitochondrial marker, ATP synthase F1 complex V β-polypeptide (ATP5B).
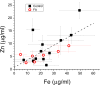
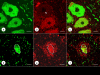
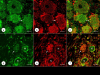
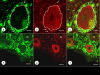
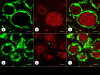
Results: XRF revealed normal total Zn- and Fe-levels in the neural tissue of DRG in FA (mean ± standard deviation): Zn=5.46±2.29 μg/ml, Fe=19.99±13.26 μg/ml in FA; Zn=8.16±6.19 μg/ml, Fe=23.85±12.23 μg/ml in controls. Despite these unchanged total metal concentrations, Zn- and Fe-related proteins displayed major shifts in their cellular localization. The Zn transporter Zip14 that is normally expressed in DRG neurons and satellite cells became more prominent in hyperplastic satellite cells and residual nodules. Metallothionein 3 (MT3) stains confirmed reduction of neuronal size in FA, but MT3 expression remained low in hyperplastic satellite cells. In contrast, MT1/2 immunofluorescence was prominent in proliferating satellite cells. Neuronal ferritin immunofluorescence declined but remained strong in hyperplastic satellite cells and residual nodules. Satellite cells in FA showed a larger number of mitochondria expressing ATB5B. Frataxin immunohistochemistry in FA confirmed small neuronal sizes, irregular distribution of reaction product beneath the plasma membrane, and enhanced expression in hyperplastic satellite cells.
Conclusions: The pool of total cellular Zn in normal DRG equals 124.8 μM, which is much higher than needed for the proper function of Zn ion-dependent proteins. It is likely that any disturbance of Zn buffering by Zip14 and MT3 causes mitochondrial damage and cell death. In contrast to Zn, sequestration of Fe in hyperplastic satellite cells may represent a protective mechanism. The changes in the cellular localization of Zn- and Fe-handling proteins suggest metal transfer from degenerating DRG neurons to activated satellite cells and connect neuronal metal dysmetabolism with the pathogenesis of the DRG lesion in FA.
Figures

References
-
- Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Cañizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;1:1423–1427. doi: 10.1126/science.271.5254.1423. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

