Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17β-estradiol's control of energy homeostasis
- PMID: 24252383
- PMCID: PMC3947695
- DOI: 10.1016/j.steroids.2013.10.018
Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17β-estradiol's control of energy homeostasis
Abstract
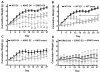
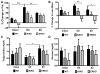
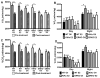
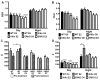
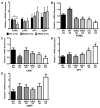
The steroid 17β-estradiol (E2) modulates energy homeostasis by reducing feeding behavior and increasing energy expenditure primarily through estrogen receptor α (ERα)-mediated mechanisms. Intact ERαKO female mice develop obesity as adults exhibiting decreased energy expenditure and increased fat deposition. However, intact transgenic female mice expressing a DNA-binding-deficient ERα (KIKO) are not obese and have similar energy expenditure, activity and fat deposition as to wild type (WT) females, suggesting that non-estrogen response element (ERE)-mediated signaling is important in E2 regulation of energy homeostasis. Initial reports did not examine the effects of ovariectomy on energy homeostasis or E2's attenuation of post-ovariectomy body weight gain. Therefore, we sought to determine if low physiological doses of E2 (250 ng QOD) known to suppress post-ovariectomy body weight gain in WT females would suppress body weight gain in ovariectomized KIKO females. We observed that the post-ovariectomy increase in body weight was significantly greater in WT females than in KIKO females. Furthermore, E2 did not significantly attenuate the body weight gain in KIKO females as it did in WT females. E2 replacement suppressed food intake and fat accumulation while increasing nighttime oxygen consumption and activity only in WT females. E2 replacement also increased arcuate POMC gene expression in WT females only. These data suggest that in the intact female, ERE-independent mechanisms are sufficient to maintain normal energy homeostasis and to partially restore the normal response to ovariectomy. However, they are not sufficient for E2's suppression of post-ovariectomy body weight gain and its effects on metabolism and activity.
Keywords: 17β-Estradiol; ERα; Energy homeostasis; Ovariectomy.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
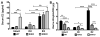
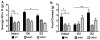
References
-
- Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. - PubMed
-
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

