Immune complex formation impairs the elimination of solutes from the brain: implications for immunotherapy in Alzheimer's disease
- PMID: 24252464
- PMCID: PMC3893559
- DOI: 10.1186/2051-5960-1-48
Immune complex formation impairs the elimination of solutes from the brain: implications for immunotherapy in Alzheimer's disease
Abstract
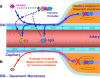
Background: Basement membranes in the walls of cerebral capillaries and arteries form a major lymphatic drainage pathway for fluid and solutes from the brain. Amyloid-β (Aβ) draining from the brain is deposited in such perivascular pathways as cerebral amyloid angiopathy (CAA) in Alzheimer's disease (AD). CAA increases in severity when Aβ is removed from the brain parenchyma by immunotherapy for AD. In this study we investigated the consequences of immune complexes in artery walls upon drainage of solutes similar to soluble Aβ. We tested the hypothesis that, following active immunization with ovalbumin, immune complexes form within the walls of cerebral arteries and impair the perivascular drainage of solutes from the brain. Mice were immunized against ovalbumin and then challenged by intracerebral microinjection of ovalbumin. Perivascular drainage of solutes was quantified following intracerebral microinjection of soluble fluorescent 3kDa dextran into the brain at different time intervals after intracerebral challenge with ovalbumin.
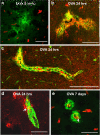
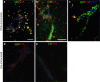
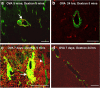
Results: Ovalbumin, IgG and complement C3 co-localized in basement membranes of artery walls 24 hrs after challenge with antigen; this was associated with significantly reduced drainage of dextran in immunized mice.
Conclusions: Perivascular drainage along artery walls returned to normal by 7 days. These results indicate that immune complexes form in association with basement membranes of cerebral arteries and interfere transiently with perivascular drainage of solutes from the brain. Immune complexes formed during immunotherapy for AD may similarly impair perivascular drainage of soluble Aβ and increase severity of CAA.
Figures



References
-
- Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. AmJ Physiol. 1981;1(4):F319–F28. - PubMed
-
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH. et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. NeuropatholApplNeurobiol. 2008;1(2):131–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

