Population-based analysis of non-steroidal anti-inflammatory drug use among children in four European countries in the SOS project: what size of data platforms and which study designs do we need to assess safety issues?
- PMID: 24252465
- PMCID: PMC4225575
- DOI: 10.1186/1471-2431-13-192
Population-based analysis of non-steroidal anti-inflammatory drug use among children in four European countries in the SOS project: what size of data platforms and which study designs do we need to assess safety issues?
Abstract
Background: Data on utilization patterns and safety of non-steroidal anti-inflammatory drugs (NSAIDs) in children are scarce. The purpose of this study was to investigate the utilization of NSAIDs among children in four European countries as part of the Safety Of non-Steroidal anti-inflammatory drugs (SOS) project.
Methods: We used longitudinal patient data from seven databases (GePaRD, IPCI, OSSIFF, Pedianet, PHARMO, SISR, and THIN) to calculate prevalence rates of NSAID use among children (0-18 years of age) from Germany, Italy, Netherlands, and United Kingdom. All databases contained a representative population sample and recorded demographics, diagnoses, and drug prescriptions. Prevalence rates of NSAID use were stratified by age, sex, and calendar time. The person-time of NSAID exposure was calculated by using the duration of the prescription supply. We calculated incidence rates for serious adverse events of interest. For these adverse events of interest, sample size calculations were conducted (alpha = 0.05; 1-beta = 0.8) to determine the amount of NSAID exposure time that would be required for safety studies in children.
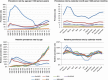
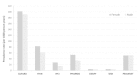
Results: The source population comprised 7.7 million children with a total of 29.6 million person-years of observation. Of those, 1.3 million children were exposed to at least one of 45 NSAIDs during observation time. Overall prevalence rates of NSAID use in children differed across countries, ranging from 4.4 (Italy) to 197 (Germany) per 1000 person-years in 2007. For Germany, United Kingdom, and Italian pediatricians, we observed high rates of NSAID use among children aged one to four years. For all four countries, NSAID use increased with older age categories for children older than 11. In this analysis, only for ibuprofen (the most frequently used NSAID), enough exposure was available to detect a weak association (relative risk of 2) between exposure and asthma exacerbation (the most common serious adverse event of interest).
Conclusions: Patterns of NSAID use in children were heterogeneous across four European countries. The SOS project platform captures data on more than 1.3 million children who were exposed to NSAIDs. Even larger data platforms and the use of advanced versions of case-only study designs may be needed to conclusively assess the safety of these drugs in children.
Figures
References
-
- Salvo F, Fourrier-Reglat A, Bazin F, Robinson P, Riera-Guardia N, Haag M, Caputi AP, Moore N, Sturkenboom MC, Pariente A. Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89:855–866. doi: 10.1038/clpt.2011.45. - DOI - PubMed
-
- Assessment of the paediatric needs, pain. Paediatric working party of the european medicines agency. 2005. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/10/WC500... (accessed April 4, 2012)
-
- World Health Organization. Classification of Diseases. Available at: http://www.who.int/classifications/icd/en/. (accessed April 4, 2011)
-
- WHO collaborating centre for drug statistics methodology. Guidelines for ATC classification and DDD assignment. Available at: http://www.whocc.no/atcddd/. (accessed April 4, 2012)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical