Dysfunction of the mTOR pathway is a risk factor for Alzheimer's disease
- PMID: 24252508
- PMCID: PMC3776211
- DOI: 10.1186/2051-5960-1-3
Dysfunction of the mTOR pathway is a risk factor for Alzheimer's disease
Abstract
Background: The development of disease-modifying therapies for Alzheimer's disease is hampered by our lack of understanding of the early pathogenic mechanisms and the lack of early biomarkers and risk factors.We have documented the expression pattern of mTOR regulated genes in the frontal cortex of Alzheimer's disease patients. We have also examined the functional integrity of mTOR signaling in peripheral lymphocytes in Alzheimer's disease patients relative to healthy controls.
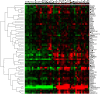
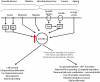
Results: In the brain mTOR is seen to control molecular functions related to cell cycle regulation, cell death and several metabolic pathways. These downstream elements of the mTOR signaling cascade are deregulated in the brain of Alzheimer's disease patients well before the development of pathology. This dysregulation of the mTOR downstream signaling cascade is not restricted to the brain but appears to be systemic and can be detected in peripheral lymphocytes as a reduced Rapamycin response.
Conclusions: The dysfunction of the signaling pathways downstream of mTOR may represent a risk factor for Alzheimer's disease and is independent of the ApoE status of the patients.We have also identified the molecular substrates of the beneficial effects of Rapamycin on the nervous system. We believe that these results can further inform the development of clinical predictive tests for the risk of Alzheimer's disease in patients with mild cognitive impairment.
Figures






References
-
- Nagy Z, Esiri MM, Smith AD. The cell division cycle and the pathophysiology of Alzheimer's disease. Neuroscience. 1998;87:731–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

