Colostral antibody-mediated and cell-mediated immunity contributes to innate and antigen-specific immunity in piglets
- PMID: 24252519
- PMCID: PMC3902642
- DOI: 10.1016/j.dci.2013.11.005
Colostral antibody-mediated and cell-mediated immunity contributes to innate and antigen-specific immunity in piglets
Abstract
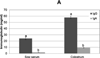
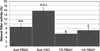
Immunoglobulins and immune cells are critical components of colostral immunity; however, their transfer to and function in the neonate, especially maternal lymphocytes, is unclear. Cell-mediated and antibody-mediated immunity in sow blood and colostrum and piglet blood before (PS) and after (AS) suckling were assessed to investigate transfer and function of maternal immunity in the piglet. CD4, CD8, and γδ lymphocytes were found in sow blood and colostrum and piglet blood PS and AS; each had a unique T lymphocyte profile. Immunoglobulins were detected in sow blood, colostrum, and in piglet blood AS; the immunoglobulin profile of piglet serum AS mimicked that of sow serum. These results suggest selectivity in lymphocyte concentration into colostrum and subsequent lymphocyte transfer into the neonate, but that immunoglobulin transfer is unimpeded. Assessment of colostral natural killer activity and antigen-specific proliferation revealed that colostral cells are capable of influencing the innate and specific immune response of neonatal pigs.
Keywords: 7-aminoactinomycin D; 7AAD; AMI; APC; AS; CFSE; CMC; CMI; Colostrum; ELISA; IgA; IgG; M. hyopneumoniae; Maternally derived immunity; Mycoplasma hyopneumoniae; NK; Neonate; PBMC; PS; Passive transfer; S:P ratio; Swine; T lymphocyte; after-suckling; antibody-mediated immunity; antigen presenting cell; carboxyfluorescein diacetate succinimidyl ester; cell-mediated immunity; colostral mononuclear cells; conA; concanavalin A; enzyme linked immunosorbant assay; immunoglobulin A; immunoglobulin G; natural killer; peripheral blood mononuclear cell; pre-suckling; sample to positive ratio.
Published by Elsevier Ltd.
Figures






References
-
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Reviews Immunol. 2004;4:553–564. - PubMed
-
- Adkins B, Chun K, Hamilton K, Nassiri M. Naive murine neonatal T cells undergo apoptosis in response to primary stimulation. J Immunol. 1996;157:1343–1349. - PubMed
-
- Bandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–268. - PubMed
-
- Baley JE, Schacter BZ. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985;134:3042–3048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

