Intrastriatal injection of interleukin-1 beta triggers the formation of neuromyelitis optica-like lesions in NMO-IgG seropositive rats
- PMID: 24252536
- PMCID: PMC3776214
- DOI: 10.1186/2051-5960-1-5
Intrastriatal injection of interleukin-1 beta triggers the formation of neuromyelitis optica-like lesions in NMO-IgG seropositive rats
Abstract
Background: Neuromyelitis optica (NMO) is a severe, disabling disease of the central nervous system (CNS) characterized by the formation of astrocyte-destructive, neutrophil-dominated inflammatory lesions in the spinal cord and optic nerves. These lesions are initiated by the binding of pathogenic aquaporin 4 (AQP4)-specific autoantibodies to astrocytes and subsequent complement-mediated lysis of these cells. Typically, these lesions form in a setting of CNS inflammation, where the blood-brain barrier is open for the entry of antibodies and complement. However, it remained unclear to which extent pro-inflammatory cytokines and chemokines contribute to the formation of NMO lesions. To specifically address this question, we injected the cytokines interleukin-1 beta, tumor necrosis factor alpha, interleukin-6, interferon gamma and the chemokine CXCL2 into the striatum of NMO-IgG seropositive rats and analyzed the tissue 24 hours later by immunohistochemistry.
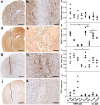
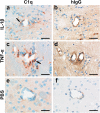
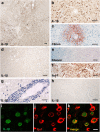
Results: All injected cytokines and chemokines led to profound leakage of immunoglobulins into the injected hemisphere, but only interleukin-1 beta induced the formation of perivascular, neutrophil-infiltrated lesions with AQP4 loss and complement-mediated astrocyte destruction distant from the needle tract. Treatment of rat brain endothelial cells with interleukin-1 beta, but not with any other cytokine or chemokine applied at the same concentration and over the same period of time, caused profound upregulation of granulocyte-recruiting and supporting molecules. Injection of interleukin-1 beta caused higher numbers of blood vessels with perivascular, cellular C1q reactivity than any other cytokine tested. Finally, the screening of a large sample of CNS lesions from NMO and multiple sclerosis patients revealed large numbers of interleukin-1 beta-reactive macrophages/activated microglial cells in active NMO lesions but not in MS lesions with comparable lesion activity and location.
Conclusions: Our data strongly suggest that interleukin-1 beta released in NMO lesions and interleukin-1 beta-induced production/accumulation of complement factors (like C1q) facilitate neutrophil entry and BBB breakdown in the vicinity of NMO lesions, and might thus be an important secondary factor for lesion formation, possibly by paving the ground for rapid lesion growth and amplified immune cell recruitment to this site.
Figures



References
-
- Devic E. Myélite subaigue compliquée de névrite optique. Bull Med. 1894;8:1033.
-
- Lennon VA, Wingerchuck DN, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;264:2106–2112. - PubMed
-
- Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, Gilden D, Nessler S, Stadelmann C, Hemmer B. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

