Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity
- PMID: 24252623
- PMCID: PMC3893391
- DOI: 10.1186/2051-5960-1-70
Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity
Abstract
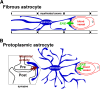
Background: Neuronal activity intimately communicates with blood flow through the blood-brain barrier (BBB) in the central nervous system (CNS). Astrocyte endfeet cover more than 90% of brain capillaries and interact with synapses and nodes of Ranvier. The roles of astrocytes in neurovascular coupling in the CNS remain poorly understood.
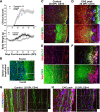
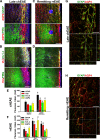
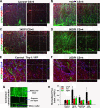
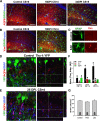
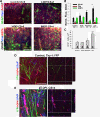
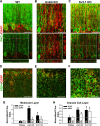
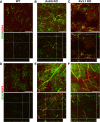
Results: Here we show that astrocytes that are intrinsically different are activated by inflammatory autoimmune insults and alterations of neuronal activity. In the progression of experimental autoimmune encephalomyelitis (EAE), both fibrous and protoplasmic astrocytes were broadly and reversibly activated in the brain and spinal cord, indicated by marked upregulation of glial fibrillary acidic protein (GFAP) and other astrocytic proteins. In early and remitting EAE, upregulated GFAP and astrocytic endfoot water channel aquaporin 4 (AQP4) enclosed white matter lesions in spinal cord, whereas they markedly increased and formed bundles in exacerbated lesions in late EAE. In cerebellar cortex, upregulation of astrocytic proteins correlated with EAE severity. On the other hand, protoplasmic astrocytes were also markedly activated in the brains of ankyrin-G (AnkG) and Kv3.1 KO mice, where neuronal activities are altered. Massive astrocytes replaced degenerated Purkinje neurons in AnkG KO mice. In Kv3.1 KO mice, GFAP staining significantly increased in cerebellar cortex, where Kv3.1 is normally highly expressed, but displayed in a patchy pattern in parts of the hippocampus.
Conclusions: Thus, astrocytes can detect changes in both blood and neurons, which supports their central role in neurovascular coupling. These studies contribute to the development of new strategies of neuroprotection and repair for various diseases, through activity-dependent regulation of neurovascular coupling.
Figures
Similar articles
-
Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation.J Immunol. 2011 Jun 1;186(11):6521-31. doi: 10.4049/jimmunol.1001135. Epub 2011 Apr 22. J Immunol. 2011. PMID: 21515788
-
The expression and activity of KV3.4 channel subunits are precociously upregulated in astrocytes exposed to Aβ oligomers and in astrocytes of Alzheimer's disease Tg2576 mice.Neurobiol Aging. 2017 Jun;54:187-198. doi: 10.1016/j.neurobiolaging.2017.03.008. Epub 2017 Mar 16. Neurobiol Aging. 2017. PMID: 28390823
-
Downregulation of water channel aquaporin-4 in rats with experimental autoimmune encephalomyeritis induced by myelin basic protein.Cell Immunol. 2013 Jan;281(1):91-9. doi: 10.1016/j.cellimm.2013.01.003. Epub 2013 Feb 8. Cell Immunol. 2013. PMID: 23510922
-
Astrocyte phenotypes: Emphasis on potential markers in neuroinflammation.Histol Histopathol. 2021 Mar;36(3):267-290. doi: 10.14670/HH-18-284. Epub 2020 Nov 23. Histol Histopathol. 2021. PMID: 33226087 Review.
-
Regulation of neurovascular coupling in autoimmunity to water and ion channels.Autoimmun Rev. 2015 Mar;14(3):258-67. doi: 10.1016/j.autrev.2014.11.010. Epub 2014 Nov 22. Autoimmun Rev. 2015. PMID: 25462580 Free PMC article. Review.
Cited by
-
The Translatability of Multiple Sclerosis Animal Models for Biomarkers Discovery and Their Clinical Use.Int J Mol Sci. 2022 Sep 29;23(19):11532. doi: 10.3390/ijms231911532. Int J Mol Sci. 2022. PMID: 36232832 Free PMC article. Review.
-
Immediate induction of varicosities by transverse compression but not uniaxial stretch in axon mechanosensation.Acta Neuropathol Commun. 2022 Jan 24;10(1):7. doi: 10.1186/s40478-022-01309-8. Acta Neuropathol Commun. 2022. PMID: 35074017 Free PMC article.
-
How does Nogo receptor influence demyelination and remyelination in the context of multiple sclerosis?Front Cell Neurosci. 2023 Jun 8;17:1197492. doi: 10.3389/fncel.2023.1197492. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37361998 Free PMC article. Review.
-
Mesial temporal lobe epilepsy with psychiatric comorbidities: a place for differential neuroinflammatory interplay.J Neuroinflammation. 2015 Feb 25;12:38. doi: 10.1186/s12974-015-0266-z. J Neuroinflammation. 2015. PMID: 25889039 Free PMC article.
-
Copper chelation and autoimmunity differentially impact myelin in the hippocampal-prefrontal circuit.J Neuroimmunol. 2019 Sep 15;334:576998. doi: 10.1016/j.jneuroim.2019.576998. Epub 2019 Jun 20. J Neuroimmunol. 2019. PMID: 31254928 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

