Cluster randomized adaptive implementation trial comparing a standard versus enhanced implementation intervention to improve uptake of an effective re-engagement program for patients with serious mental illness
- PMID: 24252648
- PMCID: PMC3874628
- DOI: 10.1186/1748-5908-8-136
Cluster randomized adaptive implementation trial comparing a standard versus enhanced implementation intervention to improve uptake of an effective re-engagement program for patients with serious mental illness
Abstract
Background: Persons with serious mental illness (SMI) are disproportionately burdened by premature mortality. This disparity is exacerbated by poor continuity of care with the health system. The Veterans Health Administration (VA) developed Re-Engage, an effective population-based outreach program to identify veterans with SMI lost to care and to reconnect them with VA services. However, such programs often encounter barriers getting implemented into routine care. Adaptive designs are needed when the implementation intervention requires augmentation within sites that do not initially respond to an initial implementation intervention. This protocol describes the methods used in an adaptive implementation design study that aims to compare the effectiveness of a standard implementation strategy (Replicating Effective Programs, or REP) with REP enhanced with External Facilitation (enhanced REP) to promote the uptake of Re-Engage.
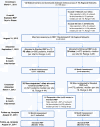
Methods/design: This study employs a four-phase, two-arm, longitudinal, clustered randomized trial design. VA sites (n = 158) across the United States with a designated Re-Engage provider, at least one Veteran with SMI lost to care, and who received standard REP during a six-month run-in phase. Subsequently, 88 sites with inadequate uptake were stratified at the cluster level by geographic region (n = 4) and VA regional service network (n = 20) and randomized to REP (n = 49) vs. enhanced REP (n = 39) in phase two. The primary outcome was the percentage of veterans on each facility outreach list documented on an electronic web registry. The intervention was at the site and network level and consisted of standard REP versus REP enhanced by external phone facilitation consults. At 12 months, enhanced REP sites returned to standard REP and 36 sites with inadequate participation received enhanced REP for six months in phase three. Secondary implementation outcomes included the percentage of veterans contacted directly by site providers and the percentage re-engaged in VA health services.
Discussion: Adaptive implementation designs consisting of a sequence of decision rules that are tailored based on a site's uptake of an effective program may produce more relevant, rapid, and generalizable results by more quickly validating or rejecting new implementation strategies, thus enhancing the efficiency and sustainability of implementation research and potentially leading to the rollout of more cost-efficient implementation strategies.
Trial registration: Current Controlled Trials ISRCTN21059161.
Figures

References
-
- McCarthy JF, Blow FC, Valenstein M, Fischer EP, Owen RR, Barry KL, Hudson TJ, Ignacio RV. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42:1042–1060. doi: 10.1111/j.1475-6773.2006.00642.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

