Alterations in immunodominance of Streptococcus mutans AgI/II: lessons learned from immunomodulatory antibodies
- PMID: 24252705
- PMCID: PMC3901311
- DOI: 10.1016/j.vaccine.2013.11.023
Alterations in immunodominance of Streptococcus mutans AgI/II: lessons learned from immunomodulatory antibodies
Abstract
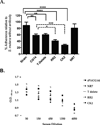
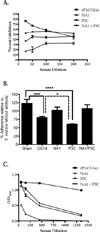
Streptococcus mutans antigen I/II (AgI/II) has been widely studied as a candidate vaccine antigen against human dental caries. In this report we follow up on prior studies that indicated that anti-AgI/II immunomodulatory monoclonal antibodies (MAbs) exerted their effects by destabilizing the native protein structure and exposing cryptic epitopes. We show here that similar results can be obtained by immunizing mice with truncated polypeptides out of the context of an intra-molecular interaction that occurs within the full-length molecule and that appears to dampen the functional response against at least two important target epitopes. Putative T cell epitopes that influenced antibody specificity were identified immediately upstream of the alanine-rich repeat domain. Adherence inhibiting antibodies could be induced against two discrete domains of the protein, one corresponding to the central portion of the molecule and the other corresponding to the C-terminus.
Keywords: Immune complex; Immunodominance; Monoclonal antibody; Streptococcus; Vaccine design.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

