Cochleovestibular nerve development is integrated with migratory neural crest cells
- PMID: 24252775
- PMCID: PMC4556368
- DOI: 10.1016/j.ydbio.2013.11.009
Cochleovestibular nerve development is integrated with migratory neural crest cells
Abstract
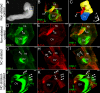
The cochleovestibular (CV) nerve, which connects the inner ear to the brain, is the nerve that enables the senses of hearing and balance. The aim of this study was to document the morphological development of the mouse CV nerve with respect to the two embryonic cells types that produce it, specifically, the otic vesicle-derived progenitors that give rise to neurons, and the neural crest cell (NCC) progenitors that give rise to glia. Otic tissues of mouse embryos carrying NCC lineage reporter transgenes were whole mount immunostained to identify neurons and NCC. Serial optical sections were collected by confocal microscopy and were compiled to render the three dimensional (3D) structure of the developing CV nerve. Spatial organization of the NCC and developing neurons suggest that neuronal and glial populations of the CV nerve develop in tandem from early stages of nerve formation. NCC form a sheath surrounding the CV ganglia and central axons. NCC are also closely associated with neurites projecting peripherally during formation of the vestibular and cochlear nerves. Physical ablation of NCC in chick embryos demonstrates that survival or regeneration of even a few individual NCC from ectopic positions in the hindbrain results in central projection of axons precisely following ectopic pathways made by regenerating NCC.
Keywords: 3-dimensional; 3D; Axon; CV; Cochlea; Craniofacial; E; Ear; GFP; Green Fluorescent Protein; NCC; Neural crest; Neuron; Otic; P; R; Vestibular; cochleovestibular; embryonic day; neural crest cells; postnatal day; rhombomere.
© 2013 Published by Elsevier Inc.
Figures

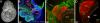



References
-
- Altman J, Bayer S. Development of the cranial nerve ganglia and related nuclei in the rat. Springer-Verlag; Berlin: 1982. - PubMed
-
- Ard MD, Morest DK, Hauger SH. Trophic interactions between the cochleovestibular ganglion of the chick embryo and its synaptic targets in culture. Neuroscience. 1985;16:151–170. - PubMed
-
- Begbie J, Ballivet M, Graham A. Early Steps in the Production of Sensory Neurons by the Neurogenic Placodes. Molecular and Cellular Neuroscience. 2002;21:502–511. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

