Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury
- PMID: 24252875
- PMCID: PMC3833342
- DOI: 10.7554/eLife.01157
Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury
Abstract
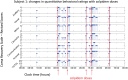
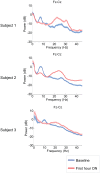
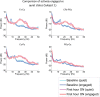
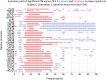
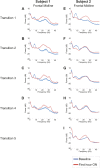
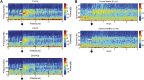
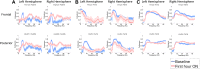
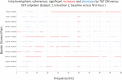
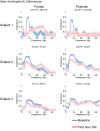
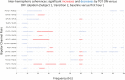
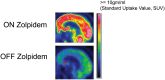
Zolpidem produces paradoxical recovery of speech, cognitive and motor functions in select subjects with severe brain injury but underlying mechanisms remain unknown. In three diverse patients with known zolpidem responses we identify a distinctive pattern of EEG dynamics that suggests a mechanistic model. In the absence of zolpidem, all subjects show a strong low frequency oscillatory peak ∼6-10 Hz in the EEG power spectrum most prominent over frontocentral regions and with high coherence (∼0.7-0.8) within and between hemispheres. Zolpidem administration sharply reduces EEG power and coherence at these low frequencies. The ∼6-10 Hz activity is proposed to arise from intrinsic membrane properties of pyramidal neurons that are passively entrained across the cortex by locally-generated spontaneous activity. Activation by zolpidem is proposed to arise from a combination of initial direct drug effects on cortical, striatal, and thalamic populations and further activation of underactive brain regions induced by restoration of cognitively-mediated behaviors. DOI: http://dx.doi.org/10.7554/eLife.01157.001.
Keywords: Consciousness; GABA-A; anesthesia; arousal; central thalamus; striatum.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

Comment in
-
Awakened by a sleeping pill.Elife. 2013 Nov 19;2:e01658. doi: 10.7554/eLife.01658. Elife. 2013. PMID: 24252876 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

