Emerging science of hydroxyurea therapy for pediatric sickle cell disease
- PMID: 24252885
- PMCID: PMC3917141
- DOI: 10.1038/pr.2013.227
Emerging science of hydroxyurea therapy for pediatric sickle cell disease
Abstract
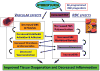
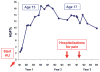
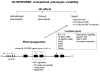
Hydroxyurea (HU) is the sole approved pharmacological therapy for sickle cell disease (SCD). Higher levels of fetal hemoglobin (HbF) diminish deoxygenated sickle globin polymerization in vitro and clinically reduce the incidence of disease morbidities. Clinical and laboratory effects of HU largely result from induction of HbF expression, though to a highly variable extent. Baseline and HU-induced HbF expression are both inherited complex traits. In children with SCD, baseline HbF remains the best predictor of drug-induced levels, but this accounts for only a portion of the induction. A limited number of validated genetic loci are strongly associated with higher baseline HbF levels in SCD. For induced HbF levels, genetic approaches using candidate single-nucleotide polymorphisms (SNPs) have identified some of these same loci as being also associated with induction. However, SNP associations with induced HbF are only partially independent of baseline levels. Additional approaches to understanding the impact of HU on HbF and its other therapeutic effects on SCD include pharmacokinetic, gene expression-based, and epigenetic analyses in patients and through studies in existing murine models for SCD. Understanding the genetic and other factors underlying the variability in therapeutic effects of HU for pediatric SCD is critical for prospectively predicting good responders and for designing other effective therapies.
Figures
References
-
- [Accessed 6 September 2012];Healthy People 2020. http://healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=4.
-
- Hassell KL. Population estimates of sickle cell disease in the U. S Am J Prev Med. 2010;38:S512–21. - PubMed
-
- Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatr Blood Cancer. 2013;60:1482–6. - PubMed
-
- Yanni E, Grosse SD, Yang Q, Olney RS. Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. J Pediatr. 2009;154:541–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

