Targeting C-myc G-quadruplex: dual recognition by aminosugar-bisbenzimidazoles with varying linker lengths
- PMID: 24252993
- PMCID: PMC6270413
- DOI: 10.3390/molecules181114228
Targeting C-myc G-quadruplex: dual recognition by aminosugar-bisbenzimidazoles with varying linker lengths
Abstract
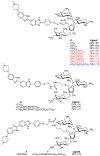
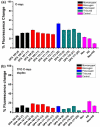
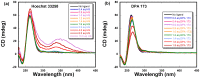
G-quadruplexes are therapeutically important biological targets. In this report, we present biophysical studies of neomycin-Hoechst 33258 conjugates binding to a G-quadruplex derived from the C-myc promoter sequence. Our studies indicate that conjugation of neomycin to a G-quadruplex binder, Hoechst 33258, enhances its binding. The enhancement in G-quadruplex binding of these conjugates varies with the length and composition of the linkers joining the neomycin and Hoechst 33258 units.
Figures
Similar articles
-
Dual recognition of the human telomeric G-quadruplex by a neomycin-anthraquinone conjugate.Chem Commun (Camb). 2013 Jun 28;49(51):5796-8. doi: 10.1039/c3cc42721h. Chem Commun (Camb). 2013. PMID: 23698792 Free PMC article.
-
Topology specific stabilization of promoter over telomeric G-quadruplex DNAs by bisbenzimidazole carboxamide derivatives.ACS Chem Biol. 2015 Mar 20;10(3):821-33. doi: 10.1021/cb5008597. Epub 2014 Dec 26. ACS Chem Biol. 2015. PMID: 25495750
-
Hoechst 33258 binds to G-quadruplex in the promoter region of human c-myc.Biochem Biophys Res Commun. 2003 Oct 17;310(2):505-12. doi: 10.1016/j.bbrc.2003.09.052. Biochem Biophys Res Commun. 2003. PMID: 14521939
-
Human MYC G-quadruplex: From discovery to a cancer therapeutic target.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188410. doi: 10.1016/j.bbcan.2020.188410. Epub 2020 Aug 19. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32827579 Review.
-
Developing Novel G-Quadruplex Ligands: from Interaction with Nucleic Acids to Interfering with Nucleic Acid⁻Protein Interaction.Molecules. 2019 Jan 22;24(3):396. doi: 10.3390/molecules24030396. Molecules. 2019. PMID: 30678288 Free PMC article. Review.
Cited by
-
Comprehensive review of chemical strategies for the preparation of new aminoglycosides and their biological activities.Chem Soc Rev. 2018 Feb 19;47(4):1189-1249. doi: 10.1039/c7cs00407a. Chem Soc Rev. 2018. PMID: 29296992 Free PMC article. Review.
-
Marine Fungi: A Source of Potential Anticancer Compounds.Front Microbiol. 2018 Jan 5;8:2536. doi: 10.3389/fmicb.2017.02536. eCollection 2017. Front Microbiol. 2018. PMID: 29354097 Free PMC article. Review.
-
A pH Sensitive High-Throughput Assay for miRNA Binding of a Peptide-Aminoglycoside (PA) Library.PLoS One. 2015 Dec 11;10(12):e0144251. doi: 10.1371/journal.pone.0144251. eCollection 2015. PLoS One. 2015. PMID: 26656788 Free PMC article.
-
New Application of Neomycin B-Bisbenzimidazole Hybrids as Antifungal Agents.ACS Infect Dis. 2018 Feb 9;4(2):196-207. doi: 10.1021/acsinfecdis.7b00254. Epub 2017 Dec 11. ACS Infect Dis. 2018. PMID: 29227087 Free PMC article.
-
A review of patents (2011-2015) towards combating resistance to and toxicity of aminoglycosides.Medchemcomm. 2016;7(1):50-68. doi: 10.1039/C5MD00453E. Epub 2015 Nov 19. Medchemcomm. 2016. PMID: 27019689 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

