New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG)
- PMID: 24253022
- PMCID: PMC4143389
- DOI: 10.1038/leu.2013.350
New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG)
Abstract
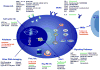
Treatment in medical oncology is gradually shifting from the use of nonspecific chemotherapeutic agents toward an era of novel targeted therapy in which drugs and their combinations target specific aspects of the biology of tumor cells. Multiple myeloma (MM) has become one of the best examples in this regard, reflected in the identification of new pathogenic mechanisms, together with the development of novel drugs that are being explored from the preclinical setting to the early phases of clinical development. We review the biological rationale for the use of the most important new agents for treating MM and summarize their clinical activity in an increasingly busy field. First, we discuss data from already approved and active agents (including second- and third-generation proteasome inhibitors (PIs), immunomodulatory agents and alkylators). Next, we focus on agents with novel mechanisms of action, such as monoclonal antibodies (MoAbs), cell cycle-specific drugs, deacetylase inhibitors, agents acting on the unfolded protein response, signaling transduction pathway inhibitors and kinase inhibitors. Among this plethora of new agents or mechanisms, some are specially promising: anti-CD38 MoAb, such as daratumumab, are the first antibodies with clinical activity as single agents in MM. Moreover, the kinesin spindle protein inhibitor Arry-520 is effective in monotherapy as well as in combination with dexamethasone in heavily pretreated patients. Immunotherapy against MM is also being explored, and probably the most attractive example of this approach is the combination of the anti-CS1 MoAb elotuzumab with lenalidomide and dexamethasone, which has produced exciting results in the relapsed/refractory setting.
Conflict of interest statement
Figures
References
-
- Greene JA, Jones DS, Podolsky SH. Therapeutic evolution and the challenge of rational medicine. N Engl J Med. 2012;367(12):1077–82. - PubMed
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–1873. - PubMed
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous