Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep
- PMID: 24253046
- PMCID: PMC3920003
- DOI: 10.1152/ajpendo.00517.2013
Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep
Abstract
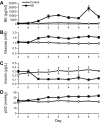
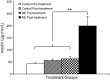
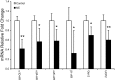
Intrauterine growth-restricted (IUGR) fetuses experience prolonged hypoxemia, hypoglycemia, and elevated norepinephrine (NE) concentrations, resulting in hypoinsulinemia and β-cell dysfunction. Previously, we showed that acute adrenergic blockade revealed enhanced insulin secretion responsiveness in the IUGR fetus. To determine whether chronic exposure to NE alone enhances β-cell responsiveness afterward, we continuously infused NE into fetal sheep for 7 days and, after terminating the infusion, evaluated glucose-stimulated insulin secretion (GSIS) and glucose-potentiated arginine-induced insulin secretion (GPAIS). During treatment, NE-infused fetuses had greater (P < 0.05) plasma NE concentrations and exhibited hyperglycemia (P < 0.01) and hypoinsulinemia (P < 0.01) compared with controls. GSIS during the NE infusion was also reduced (P < 0.05) compared with pretreatment values. GSIS and GPAIS were approximately fourfold greater (P < 0.01) in NE fetuses 3 h after the 7 days that NE infusion was discontinued compared with age-matched controls or pretreatment GSIS and GPAIS values of NE fetuses. In isolated pancreatic islets from NE fetuses, mRNA concentrations of adrenergic receptor isoforms (α1D, α2A, α2C, and β1), G protein subunit-αi-2, and uncoupling protein 2 were lower (P < 0.05) compared with controls, but β-cell regulatory genes were not different. Our findings indicate that chronic exposure to elevated NE persistently suppresses insulin secretion. After removal, NE fetuses demonstrated a compensatory enhancement in insulin secretion that was associated with adrenergic desensitization and greater stimulus-secretion coupling in pancreatic islets.
Keywords: adrenergic receptor; catecholamines; intrauterine growth restriction; uncoupling protein 2; β-cell.
Figures

References
-
- Aldoretta PW, Carver TD, Hay WW., Jr. Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate 73: 375–386, 1998 - PubMed
-
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci 11: 97–118, 1988 - PubMed
-
- Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am J Physiol Regul Integr Comp Physiol 274: R1536–R1545, 1998 - PubMed
-
- Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol 9: 17–29, 1987 - PubMed
-
- Benovic JL, Onorato JJ, Caron MG, Lefkowitz RJ. Regulation of G protein-coupled receptors by agonist-dependent phosphorylation. Soc Gen Physiol Ser 45: 87–103, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

