Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy
- PMID: 24253250
- PMCID: PMC3905756
- DOI: 10.1093/cid/cit748
Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy
Abstract
Background: Statins, or 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, have anti-inflammatory effects that are independent of their lipid-lowering properties. Despite suppressive antiretroviral therapy (ART), elevated levels of immune activation and inflammation often persist.
Methods: The Stopping Atherosclerosis and Treating Unhealthy Bone With Rosuvastatin in HIV (SATURN-HIV) trial is a randomized, double-blind, placebo-controlled study, designed to investigate the effects of rosuvastatin (10 mg/daily) on markers of cardiovascular disease risk in ART-treated human immunodeficiency virus (HIV)-infected subjects. A preplanned analysis was to assess changes in markers of immune activation at week 24. Subjects with low-density lipoprotein cholesterol <130 mg/dL and heightened immune activation (%CD8(+)CD38(+)HLA-DR(+) ≥19%, or plasma high-sensitivity C-reactive protein ≥2 mg/L) were randomized to receive rosuvastatin or placebo. We measured plasma (soluble CD14 and CD163) and cellular markers of monocyte activation (proportions of monocyte subsets and tissue factor expression) and T-cell activation (expression of CD38, HLA-DR, and PD1).
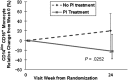
Results: After 24 weeks of rosuvastatin, we found significant decreases in plasma levels of soluble CD14 (-13.4% vs 1.2%, P = .002) and in proportions of tissue factor-positive patrolling (CD14(Dim)CD16(+)) monocytes (-38.8% vs -11.9%, P = .04) in rosuvastatin-treated vs placebo-treated subjects. These findings were independent of the lipid-lowering effect and the use of protease inhibitors. Rosuvastatin did not lead to any changes in levels of T-cell activation.
Conclusions: Rosuvastatin treatment effectively lowered markers of monocyte activation in HIV-infected subjects on antiretroviral therapy.
Clinical trials registration: NCT01218802.
Keywords: HIV-1; monocytes; rosuvastatin; tissue factor.
Figures

References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. - PubMed
-
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. - PubMed
-
- Hsue PY, Giri K, Erickson S, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109:316–9. - PubMed
-
- Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8. - PubMed
-
- Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000;11:41–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

