Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses
- PMID: 24253287
- PMCID: PMC3952667
- DOI: 10.1093/infdis/jit609
Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses
Abstract
Background: Middle East respiratory syndrome coronavirus (MERS-CoV) emerged in 2012, causing severe acute respiratory disease and pneumonia, with 44% mortality among 136 cases to date. Design of vaccines to limit the virus spread or diagnostic tests to track newly emerging strains requires knowledge of antigenic and serologic relationships between MERS-CoV and other CoVs.
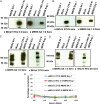
Methods: Using synthetic genomics and Venezuelan equine encephalitis virus replicons (VRPs) expressing spike and nucleocapsid proteins from MERS-CoV and other human and bat CoVs, we characterize the antigenic responses (using Western blot and enzyme-linked immunosorbent assay) and serologic responses (using neutralization assays) against 2 MERS-CoV isolates in comparison with those of other human and bat CoVs.
Results: Serologic and neutralization responses against the spike glycoprotein were primarily strain specific, with a very low level of cross-reactivity within or across subgroups. CoV N proteins within but not across subgroups share cross-reactive epitopes with MERS-CoV isolates. Our findings were validated using a convalescent-phase serum specimen from a patient infected with MERS-CoV (NA 01) and human antiserum against SARS-CoV, human CoV NL63, and human CoV OC43.
Conclusions: Vaccine design for emerging CoVs should involve chimeric spike protein containing neutralizing epitopes from multiple virus strains across subgroups to reduce immune pathology, and a diagnostic platform should include a panel of nucleocapsid and spike proteins from phylogenetically distinct CoVs.
Keywords: Diagnostics; MERS-CoV Vaccine Design; Serology; Synthetic Genomics.
Figures





References
-
- Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

