A novel molecular pathway for Snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis
- PMID: 24253315
- PMCID: PMC3919444
- DOI: 10.1158/1940-6207.CAPR-13-0263
A novel molecular pathway for Snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis
Abstract
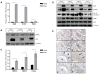
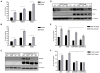
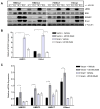
Definition of the molecular pathogenesis of lung cancer allows investigators an enhanced understanding of the natural history of the disease, thus fostering development of new prevention strategies. In addition to regulating epithelial-to-mesenchymal transition (EMT), the transcription factor Snail exerts global effects on gene expression. Our recent studies reveal that Snail is upregulated in non-small cell lung cancer (NSCLC), is associated with poor prognosis, and promotes tumor progression in vivo. Herein, we demonstrate that overexpression of Snail leads to the upregulation of secreted protein, acidic and rich in cysteine (SPARC) in models of premalignancy and established disease, as well as in lung carcinoma tissues in situ. Snail overexpression leads to increased SPARC-dependent invasion in vitro, indicating that SPARC may play a role in lung cancer progression. Bioinformatic analysis implicates transforming growth factor beta (TGF-β), extracellular signal-regulated kinase (ERK)1/2, and miR-29b as potential intermediaries in Snail-mediated upregulation of SPARC. Both the TGF-β1 ligand and TGF-β receptor 2 (TGF-βR2) are upregulated following Snail overexpression. Treatment of human bronchial epithelial cell (HBEC) lines with TGF-β1 and inhibition of TGF-β1 mRNA expression modulates SPARC expression. Inhibition of MAP-ERK kinase (MEK) phosphorylation downregulates SPARC. MiR-29b is downregulated in Snail-overexpressing cell lines, whereas overexpression of miR-29b inhibits SPARC expression. In addition, miR-29b is upregulated following ERK inhibition, suggesting a Snail-dependent pathway by which Snail activation of TGF-β and ERK signaling results in downregulation of miR-29b and subsequent upregulation of SPARC. Our discovery of pathways responsible for Snail-induced SPARC expression contributes to the definition of NSCLC pathogenesis.
©2013 AACR.
Conflict of interest statement
Figures


Similar articles
-
JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells.Int J Oncol. 2014 May;44(5):1643-51. doi: 10.3892/ijo.2014.2310. Epub 2014 Feb 21. Int J Oncol. 2014. PMID: 24573038
-
SPARC is a key mediator of TGF-β-induced renal cancer metastasis.J Cell Physiol. 2021 Mar;236(3):1926-1938. doi: 10.1002/jcp.29975. Epub 2020 Aug 11. J Cell Physiol. 2021. PMID: 32780451
-
MicroRNA-527 inhibits TGF-β/SMAD induced epithelial-mesenchymal transition via downregulating SULF2 expression in non-small-cell lung cancer.Math Biosci Eng. 2019 May 23;16(5):4607-4621. doi: 10.3934/mbe.2019231. Math Biosci Eng. 2019. PMID: 31499680
-
Analysing the relevance of TGF-β and its regulators in cervical cancer to identify therapeutic and diagnostic markers.Gene. 2025 Feb 20;938:149166. doi: 10.1016/j.gene.2024.149166. Epub 2024 Dec 18. Gene. 2025. PMID: 39701195 Review.
-
Omics Overview of the SPARC Gene in Mesothelioma.Biomolecules. 2023 Jul 11;13(7):1103. doi: 10.3390/biom13071103. Biomolecules. 2023. PMID: 37509139 Free PMC article. Review.
Cited by
-
Prognostic significance of SPARC expression in non-small cell lung cancer: A meta-analysis and bioinformatics analysis.Oncol Lett. 2022 Sep 27;24(5):412. doi: 10.3892/ol.2022.13532. eCollection 2022 Nov. Oncol Lett. 2022. PMID: 36245823 Free PMC article.
-
The cnidarian origin of the proto-oncogenes NF-κB/STAT and WNT-like oncogenic pathway drives the ctenophores (Review).Int J Oncol. 2015 Oct;47(4):1211-29. doi: 10.3892/ijo.2015.3102. Epub 2015 Jul 23. Int J Oncol. 2015. PMID: 26239915 Free PMC article. Review.
-
Secreted protein acidic and rich in cysteine (SPARC) induces cell migration and epithelial mesenchymal transition through WNK1/snail in non-small cell lung cancer.Oncotarget. 2017 Jul 22;8(38):63691-63702. doi: 10.18632/oncotarget.19475. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28969021 Free PMC article.
-
IKKα Promotes the Progression and Metastasis of Non-Small Cell Lung Cancer Independently of its Subcellular Localization.Comput Struct Biotechnol J. 2019 Feb 7;17:251-262. doi: 10.1016/j.csbj.2019.02.003. eCollection 2019. Comput Struct Biotechnol J. 2019. PMID: 30867890 Free PMC article.
-
SPARC: a potential target for functional nanomaterials and drugs.Front Mol Biosci. 2023 Jul 28;10:1235428. doi: 10.3389/fmolb.2023.1235428. eCollection 2023. Front Mol Biosci. 2023. PMID: 37577749 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan;63(1):11–30. Epub 2013/01/22.eng. - PubMed
-
- Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr Opin Pulm Med. 2009 Jul;15(4):303–7. Epub 2009/05/07.eng. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74. Epub 2011/03/08.eng. - PubMed
-
- Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006 May 15;66(10):5338–45. Epub 2006/05/19.eng. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

