Rare mutations in renal sodium and potassium transporter genes exhibit impaired transport function
- PMID: 24253496
- PMCID: PMC4007692
- DOI: 10.1097/01.mnh.0000437204.84826.99
Rare mutations in renal sodium and potassium transporter genes exhibit impaired transport function
Abstract
Purpose of review: Recent efforts to explore the genetic underpinnings of hypertension revealed rare mutations in kidney salt transport genes contribute to blood pressure (BP) variation and hypertension susceptibility in the general population. The current review focuses on these latest findings, highlighting a discussion about the rare mutations and how they affect the transport function.
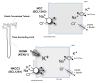
Recent findings: Rare mutations that confer a low BP trait and resistance to hypertension have recently been extensively studied. Physiological and biochemical analyses of the affected renal salt transport molecules [NKCC2 (SLC12A1), ROMK (KCNJ1), and NCC (SLC12A3)] revealed that most of the mutations do, in fact, cause a loss of transport function. The mutations disrupt the transport by many different mechanisms, including altering biosynthetic processing, trafficking, ion transport, and regulation.
Summary: New insights into the genetic basis of hypertension have recently emerged, supporting a major role of rare, rather than common, gene variants. Many different rare mutations have been found to affect the functions of different salt transporter genes by different mechanisms, yet all confer the same BP phenotype. These studies reinforce the critical roles of the kidney, and renal salt transport in BP regulation and hypertension.
Conflict of interest statement
Conflicts of Interest: PAW served as a paid consultant for Pfizer and Bristol Myers Squib in the last year.
Figures


References
-
- Salvi E, Kutalik Z, Glorioso N, et al. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension. 2012 Feb;59(2):248–55. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

