Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance
- PMID: 24253566
- PMCID: PMC4049931
- DOI: 10.4161/gmic.26854
Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance
Abstract
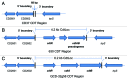
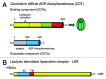
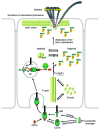
Binary toxin (CDT) is frequently observed in Clostridium difficile strains associated with increased severity of C. difficile infection (CDI). CDT belongs to the family of binary ADP-ribosylating toxins consisting of two separate toxin components: CDTa, the enzymatic ADP-ribosyltransferase which modifies actin, and CDTb which binds to host cells and translocates CDTa into the cytosol. CDTb is activated by serine proteases and binds to lipolysis stimulated lipoprotein receptor. ADP-ribosylation induces depolymerization of the actin cytoskeleton. Toxin-induced actin depolymerization also produces microtubule-based membrane protrusions which form a network on epithelial cells and increase bacterial adherence. Multiple clinical studies indicate an association between binary toxin genes in C. difficile and increased 30-d CDI mortality independent of PCR ribotype. Further studies including measures of binary toxin in stool, analyses of CDI mortality caused by CDT-producing strains, and examination of the relationship of CDT expression to TcdA and TcdB toxin variants and PCR ribotypes are needed.
Keywords: CDT; Clostridium difficileinfection; PCR ribotyping; binary toxin; disease severity; mechanism; mortality; toxinotyping.
Figures

References
-
- Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–9. doi: 10.1056/NEJMoa051639. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
