Structure-function relationships of membrane-associated GT-B glycosyltransferases
- PMID: 24253765
- PMCID: PMC3907083
- DOI: 10.1093/glycob/cwt101
Structure-function relationships of membrane-associated GT-B glycosyltransferases
Abstract
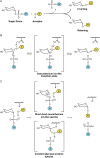
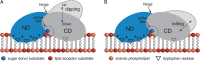
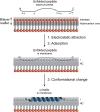
Membrane-associated GT-B glycosyltransferases (GTs) comprise a large family of enzymes that catalyze the transfer of a sugar moiety from nucleotide-sugar donors to a wide range of membrane-associated acceptor substrates, mostly in the form of lipids and proteins. As a consequence, they generate a significant and diverse amount of glycoconjugates in biological membranes, which are particularly important in cell-cell, cell-matrix and host-pathogen recognition events. Membrane-associated GT-B enzymes display two "Rossmann-fold" domains separated by a deep cleft that includes the catalytic center. They associate permanently or temporarily to the phospholipid bilayer by a combination of hydrophobic and electrostatic interactions. They have the remarkable property to access both hydrophobic and hydrophilic substrates that reside within chemically distinct environments catalyzing their enzymatic transformations in an efficient manner. Here, we discuss the considerable progress that has been made in recent years in understanding the molecular mechanism that governs substrate and membrane recognition, and the impact of the conformational transitions undergone by these GTs during the catalytic cycle.
Keywords: X-ray crystallography; carbohydrate-modifying enzyme; glycosyltransferase; membrane protein; structural biology.
Figures
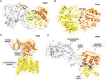

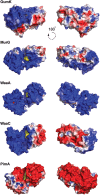
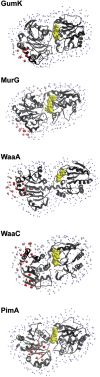
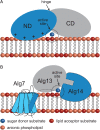

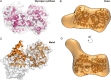
References
-
- Abdian PL, Lellouch AC, Gautier C, Ielpi L, Geremia RA. Identification of essential amino acids in the bacterial alpha-mannosyltransferase AceA. J Biol Chem. 2000;275:40568–40575. doi:10.1074/jbc.M007496200. - DOI - PubMed
-
- Andersen OS, Koeppe RE., II Bilayer thickness and membrane protein function: An energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi:10.1146/annurev.biophys.36.040306.132643. - DOI - PubMed
-
- Andersson AS, Rilfors L, Bergqvist M, Persson S, Lindblom G. New aspects on membrane lipid regulation in Acholeplasma laidlawii A and phase equilibria of monoacyl-diglucosyldiacylglycerol. Biochemistry. 1996;35:11119–11130. doi:10.1021/bi960561w. - DOI - PubMed
-
- Andrés E, Biarnés X, Faijes M, Planas A. Bacterial glycoglycerolipid synthases: Processive and non-processive glycosyltransferases in mycoplasma. Biocatal Biotransfor. 2012;30:274–287. doi:10.3109/10242422.2012.674733. - DOI
-
- Ardévol A, Rovira C. The molecular mechanism of enzymatic glycosyl transfer with retention of configuration: Evidence for a short-lived oxocarbenium-like species. Angew Chem Int Ed Engl. 2011;50:10897–10901. doi:10.1002/anie.201104623. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

