Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone
- PMID: 24253831
- PMCID: PMC3963539
- DOI: 10.1136/thoraxjnl-2013-203600
Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone
Abstract
Background: Combination therapy with an inhaled corticosteroid (ICS) and long-acting β2 agonist (LABA) is recommended for patients with asthma symptomatic on ICS alone. However, there is ongoing debate regarding the risk-benefit ratio of using LABA in asthma.
Objective: To evaluate the effect of the addition of a novel LABA, vilanterol (VI), to a once-daily ICS, fluticasone furoate (FF), on the risk of severe asthma exacerbations in patients with uncontrolled asthma.
Methods: This randomised double-blind comparative study of variable duration (≥ 24-78 weeks) was designed to finish after 330 events (each patient's first on-treatment severe asthma exacerbation). 2019 patients with asthma aged ≥ 12 years with ≥ 1 recorded exacerbation within 1 year were randomised and received FF/VI 100/25 μg or FF 100 μg, administered once daily in the evening. The primary endpoint was time to first severe exacerbation; secondary endpoints were rate of severe asthma exacerbations per patient per year and change in trough evening forced expiratory volume in 1 s (FEV1) from baseline.
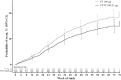
Results: Compared with FF, FF/VI delayed the time to first severe exacerbation (HR 0.795, 95% CI 0.642 to 0.985) and reduced the annualised rate of severe exacerbations (rate reduction 25%, 95% CI 5% to 40%). Significantly greater improvements in trough FEV1 (p<0.001) were observed with FF/VI than with FF at weeks 12, 36, 52 and at endpoint. Both treatments were well tolerated with similar rates of treatment-related adverse events and on-treatment serious adverse events.
Conclusions: Once-daily FF/VI reduced the risk of severe asthma exacerbations and improved lung function compared with FF alone, with good tolerability and safety profile in adolescents and adults with asthma currently receiving ICS.
Clinicaltrialsgov no: NCT01086384.
Keywords: Asthma; Asthma Pharmacology.
Figures



References
-
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. Updated 2011. http://www.ginasthma.org/uploads/users/files/GINA_Report_2011.pdf (accessed 8 Jul 2013)
-
- O'Byrne PM, Naya IP, Kallen A, et al. Increasing doses of inhaled corticosteroids compared to adding long-acting β2-agonists in achieving asthma control. Chest 2008;134:1192–9 - PubMed
-
- Shapiro G, Lumry W, Wolfe J, et al. Combined salmeterol 50 µg and fluticasone propionate 250 µg in the Diskus device for the treatment of asthma. Am J Respir Crit Care Med 2000;161:527–34 - PubMed
-
- Nelson HS, Weiss ST, Bleecker ER, et al. The salmeterol multicenter asthma research trial. Chest 2006;129:15–26 - PubMed
-
- Salpeter SR, Buckley NS, Ormiston TM, et al. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med 2006;144:904–12 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
