Memory T Cells Mediate Cardiac Allograft Vasculopathy and are Inactivated by Anti-OX40L Monoclonal Antibody
- PMID: 24254032
- PMCID: PMC4539019
- DOI: 10.1007/s10557-013-6502-9
Memory T Cells Mediate Cardiac Allograft Vasculopathy and are Inactivated by Anti-OX40L Monoclonal Antibody
Abstract
Purpose: Cardiac allograft vasculopathy (CAV) is a major complication limiting the long-term survival of cardiac transplants. The role of memory T cells (Tmem) in the pathogenesis of CAV remains elusive. This study investigated the role of Tmem cells in the development of CAV and the therapeutic potential of targeting the OX40/OX40L pathway for heart transplant survival.
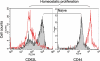
Methods: Tmem cells were generated in Rag-1(-/-) C57BL/6 (B6) mice by homeostatic proliferation (HP) of CD40L null CD3(+) T cells from B6 mice. Rag-1(-/-) B6 mice (H-2(b)) harboring Tmem cells received cardiac allografts from BALB/c mice (H-2(d)), and were either untreated or treated with anti-OX40L monoclonal antibody (mAb) (0.5 mg/mouse/day) for 10 days.
Results: Six weeks after HP, the majority of transferred CD40L(-/-) T cells in Rag-1(-/-) B6 mice were differentiated to CD44(high) and CD62L(low) Tmem cells. BALB/c heart allografts in Rag-1(-/-) B6 recipient mice in the presence of these Tmem cells developed a typical pathological feature of CAV; intimal thickening, 100 days after transplantation. However, functionally blocking the OX40/OX40L pathway with anti-OX40L mAb significantly prevented CAV development and reduced the Tmem cell population in recipient mice. Anti-OX40L mAb therapy also significantly decreased cellular infiltration and cytokine (IFN-γ, TNF-α and TGF-β) expression in heart allografts.
Conclusions: Tmem cells mediate CAV in heart transplants. Functionally blocking the OX40/OX40L pathway using anti-OX40L mAb therapy prevents Tmem cell-mediated CAV, suggesting therapeutic potential for disrupting OX40-OX40L signaling in order to prevent CAV in heart transplant patients.
Figures




Comment in
-
Tmem time: memory T-cells in cardiac allograft vasculopathy : editorial to: "memory t cells mediate cardiac allograft vasculopathy and are inactivated by anti-OX40L monoclonal antibody" by Hao Wang et al.Cardiovasc Drugs Ther. 2014 Apr;28(2):111-2. doi: 10.1007/s10557-013-6507-4. Cardiovasc Drugs Ther. 2014. PMID: 24384980 Free PMC article. No abstract available.
References
-
- Murphy L, Pinney SP. Clinical outcomes following heart transplantation. Mt Sinai J Med. 2012;79(3):317–29. - PubMed
-
- Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report–2007. J Heart Lung Transplant. 2007;26(8):769–81. - PubMed
-
- Gaudin PB, Rayburn BK, Hutchins GM, Kasper EK, Baughman KL, Goodman SN, et al. Peritransplant injury to the myocardium associated with the development of accelerated arteriosclerosis in heart transplant recipients. Am J Surg Pathol. 1994;18(4):338–46. - PubMed
-
- Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99(8):801–15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

