Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein abnormal INFLORESCENCE MERISTEM are required for the production of benzoylated metabolites in Arabidopsis seeds
- PMID: 24254312
- PMCID: PMC3875823
- DOI: 10.1104/pp.113.229807
Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein abnormal INFLORESCENCE MERISTEM are required for the production of benzoylated metabolites in Arabidopsis seeds
Abstract
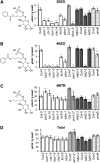
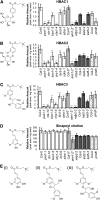
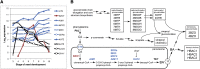
Secondary metabolites derived from benzoic acid (BA) are of central importance in the interactions of plants with pests, pathogens, and symbionts and are potentially important in plant development. Peroxisomal β-oxidation has recently been shown to contribute to BA biosynthesis in plants, but not all of the enzymes involved have been defined. In this report, we demonstrate that the peroxisomal ATP-binding cassette transporter COMATOSE is required for the accumulation of benzoylated secondary metabolites in Arabidopsis (Arabidopsis thaliana) seeds, including benzoylated glucosinolates and substituted hydroxybenzoylcholines. The ABNORMAL INFLORESCENCE MERISTEM protein, one of two multifunctional proteins encoded by Arabidopsis, is essential for the accumulation of these compounds, and MULTIFUNCTIONAL PROTEIN2 contributes to the synthesis of substituted hydroxybenzoylcholines. Of the two major 3-ketoacyl coenzyme A thiolases, KAT2 plays the primary role in BA synthesis. Thus, BA biosynthesis in Arabidopsis employs the same core set of β-oxidation enzymes as in the synthesis of indole-3-acetic acid from indole-3-butyric acid.
Figures
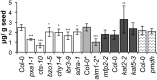
References
-
- Böttcher C, von Roepenack-Lahaye E, Schmidt J, Clemens S, Scheel D. (2009) Analysis of phenolic choline esters from seeds of Arabidopsis thaliana and Brassica napus by capillary liquid chromatography/electrospray-tandem mass spectrometry. J Mass Spectrom 44: 466–476 - PubMed
-
- Burow M, Müller R, Gershenzon J, Wittstock U. (2006) Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J Chem Ecol 32: 2333–2349 - PubMed
-
- Colquhoun TA, Marciniak DM, Wedde AE, Kim JY, Schwieterman ML, Levin LA, Van Moerkercke A, Schuurink RC, Clark DG. (2012) A peroxisomally localized acyl-activating enzyme is required for volatile benzenoid formation in a Petunia × hybrida cv. ‘Mitchell Diploid’ flower. J Exp Bot 63: 4821–4833 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

