Superior proteome stability in the longest lived animal
- PMID: 24254744
- PMCID: PMC4082568
- DOI: 10.1007/s11357-013-9597-9
Superior proteome stability in the longest lived animal
Abstract
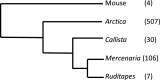
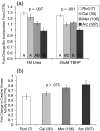
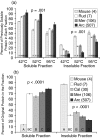
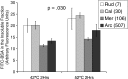
Bivalve mollusks have several unique traits, including some species with exceptionally long lives, others with very short lives, and the ability to determine the age of any individual from growth rings in the shell. Exceptionally long-lived species are seldom studied yet have the potential to be particularly informative with respect to senescence-resistance mechanisms. To this end, we employed a range of marine bivalve mollusk species, with lifespans ranging from under a decade to over 500 years, in a comparative study to investigate the hypothesis that long life requires superior proteome stability. This experimental system provides a unique opportunity to study closely related organisms with vastly disparate longevities, including the longest lived animal, Arctica islandica.Specifically, we investigated relative ability to protect protein structure and function, both basally and under various stressors in our range of species. We found a consistent relationship between species longevity, resistance to protein unfolding, and maintenance of endogenous enzyme (creatine kinase) activity. Remarkably, our longest-lived species, Arctica islandica (maximum longevity >500 years), had no increase in global proteome unfolding in response to several stressors. Additionally, the global proteome of shorter-lived species exhibited less resistance to temperature-induced protein aggregation than longer-lived species. A reporter assay, in which the same protein's aggregation properties was assessed in lysates from each study species, suggests that some endogenous feature in the cells of long-lived species, perhaps small molecular chaperones, was at least partially responsible for their enhanced proteome stability. This study reinforces the relationship between proteostasis and longevity through assessment of unfolding, function, and aggregation in species ranging in longevity from less than a decade to more than five centuries.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
