Allelic specificity of Ube3a expression in the mouse brain during postnatal development
- PMID: 24254964
- PMCID: PMC3984624
- DOI: 10.1002/cne.23507
Allelic specificity of Ube3a expression in the mouse brain during postnatal development
Abstract
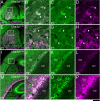
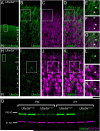
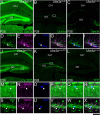
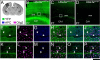
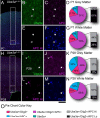
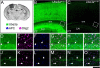
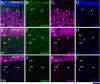
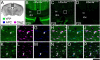
Genetic alterations of the maternal UBE3A allele result in Angelman syndrome (AS), a neurodevelopmental disorder characterized by severe developmental delay, lack of speech, and difficulty with movement and balance. The combined effects of maternal UBE3A mutation and cell type-specific epigenetic silencing of paternal UBE3A are hypothesized to result in a complete loss of functional UBE3A protein in neurons. However, the allelic specificity of UBE3A expression in neurons and other cell types in the brain has yet to be characterized throughout development, including the early postnatal period when AS phenotypes emerge. Here we define maternal and paternal allele-specific Ube3a protein expression throughout postnatal brain development in the mouse, a species that exhibits orthologous epigenetic silencing of paternal Ube3a in neurons and AS-like behavioral phenotypes subsequent to maternal Ube3a deletion. We find that neurons downregulate paternal Ube3a protein expression as they mature and, with the exception of neurons born from postnatal stem cell niches, do not express detectable paternal Ube3a beyond the first postnatal week. By contrast, neurons express maternal Ube3a throughout postnatal development, during which time localization of the protein becomes increasingly nuclear. Unlike neurons, astrocytes and oligodendrotyes biallelically express Ube3a. Notably, mature oligodendrocytes emerge as the predominant Ube3a-expressing glial cell type in the cortex and white matter tracts during postnatal development. These findings demonstrate the spatiotemporal characteristics of allele-specific Ube3a expression in key brain cell types, thereby improving our understanding of the developmental parameters of paternal Ube3a silencing and the cellular basis of AS.
Keywords: AS model mice; Angelman syndrome; E6-AP; epigenetic silencing; genomic imprinting.
Copyright © 2013 Wiley Periodicals, Inc.
Figures





References
-
- Albrecht U, Sutcliffe JS, Cattanach BM, Beechey CV, Armstrong D, Eichele G, Beaudet AL. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nature genetics. 1997;17(1):75–78. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. The Journal of comparative neurology. 1966;126(3):337–389. - PubMed
-
- Angevine JB, Jr., Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
-
- Bayer SA, Altman J. The effects of X-irradiation on the postnatally-forming granule cell populations in the olfactory bulb, hippocampus, and cerebellum of the rat. Experimental neurology. 1975;48(1):167–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

