Enhanced wound healing, kinase and stem cell marker expression in diabetic organ-cultured human corneas upon MMP-10 and cathepsin F gene silencing
- PMID: 24255036
- PMCID: PMC3867183
- DOI: 10.1167/iovs.13-13233
Enhanced wound healing, kinase and stem cell marker expression in diabetic organ-cultured human corneas upon MMP-10 and cathepsin F gene silencing
Abstract
Purpose: Diabetic corneas overexpress proteinases including matrix metalloproteinase-10 (M10) and cathepsin F (CF). Our purpose was to assess if silencing M10 and CF in organ-cultured diabetic corneas using recombinant adenovirus (rAV)-driven small hairpin RNA (rAV-sh) would normalize slow wound healing, and diabetic and stem cell marker expression.
Methods: Sixteen pairs of organ-cultured autopsy human diabetic corneas (four per group) were treated with rAV-sh. Proteinase genes were silenced either separately, together, or both, in combination (Combo) with rAV-driven c-met gene overexpression. Fellow control corneas received rAV-EGFP. Quantitative RT-PCR confirmed small hairpin RNA (shRNA) silencing effect. Ten days after transfection, 5-mm epithelial wounds were made with n-heptanol and healing time recorded. Diabetic, signaling, and putative stem cell markers were studied by immunofluorescence of corneal cryostat sections.
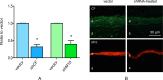
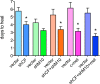
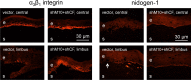
Results: Proteinase silencing reduced epithelial wound healing time versus rAV-enhanced green fluorescent protein (EGFP) control (23% for rAV-shM10, 31% for rAV-shCF, and 36% for rAV-shM10 + rAV-shCF). Combo treatment was even more efficient (55% reduction). Staining patterns of diabetic markers (α₃β₁ integrin and nidogen-1), and of activated epidermal growth factor receptor and its signaling target activated Akt were normalized upon rAV-sh treatment. Combo treatment also restored normal staining for activated p38. All treatments, especially the combined ones, increased diabetes-altered staining for putative limbal stem cell markers, ΔNp63α, ABCG2, keratins 15 and 17, and laminin γ3 chain.
Conclusions: Small hairpin RNA silencing of proteinases overexpressed in diabetic corneas enhanced corneal epithelial and stem cell marker staining and accelerated wound healing. Combined therapy with c-met overexpression was even more efficient. Specific corneal gene therapy has a potential for treating diabetic keratopathy.
Keywords: Akt; EGFR; MMP-10; c-met; cathepsin F; diabetic cornea; gene therapy; keratin; limbal stem cell; organ culture; p-38; wound healing.
Figures


References
-
- Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998; 21: 143–156 - PubMed
-
- Nakamura M, Kanamori A, Negi A. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica. 2005; 219: 1–10 - PubMed
-
- Blum M, Kloos C, Müller N, et al. Prevalence of diabetic retinopathy. Check-up program of a public health insurance company in Germany 2002–2004 [in German]. Ophthalmologe. 2007; 104: 499–500; 502–504 - PubMed
-
- Helbig H. Surgery for diabetic retinopathy. Ophthalmologica. 2007; 221: 103–111 - PubMed
-
- Murtha T, Cavallerano J. The management of diabetic eye disease in the setting of cataract surgery. Curr Opin Ophthalmol. 2007; 18: 13–18 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

