Cell-intrinsic drivers of dendrite morphogenesis
- PMID: 24255095
- PMCID: PMC3833426
- DOI: 10.1242/dev.087676
Cell-intrinsic drivers of dendrite morphogenesis
Abstract
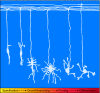
The proper formation and morphogenesis of dendrites is fundamental to the establishment of neural circuits in the brain. Following cell cycle exit and migration, neurons undergo organized stages of dendrite morphogenesis, which include dendritic arbor growth and elaboration followed by retraction and pruning. Although these developmental stages were characterized over a century ago, molecular regulators of dendrite morphogenesis have only recently been defined. In particular, studies in Drosophila and mammalian neurons have identified numerous cell-intrinsic drivers of dendrite morphogenesis that include transcriptional regulators, cytoskeletal and motor proteins, secretory and endocytic pathways, cell cycle-regulated ubiquitin ligases, and components of other signaling cascades. Here, we review cell-intrinsic drivers of dendrite patterning and discuss how the characterization of such crucial regulators advances our understanding of normal brain development and pathogenesis of diverse cognitive disorders.
Keywords: Cell-intrinsic driver; Dendrite development; Dendrite morphogenesis; Dendrite patterning; Transcription factor; Ubiquitin ligases.
Figures

References
-
- Aizawa H., Hu S. C., Bobb K., Balakrishnan K., Ince G., Gurevich I., Cowan M., Ghosh A. (2004). Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science 303, 197–202 - PubMed
-
- Armstrong D. D. (1995). The neuropathology of Rett syndrome—overview 1994. Neuropediatrics 26, 100–104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

