A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure
- PMID: 24255129
- PMCID: PMC3924183
- DOI: 10.1093/eurheartj/eht459
A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure
Abstract
Aims: The aim of this study was to evaluate the haemodynamic effects of serelaxin (30 µg/kg/day 20-h infusion and 4-h post-infusion period) in patients with acute heart failure (AHF).
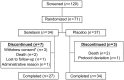
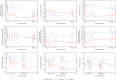
Methods and results: This double-blind, multicentre study randomized 71 AHF patients with pulmonary capillary wedge pressure (PCWP) ≥ 18 mmHg, systolic blood pressure (BP) ≥ 115 mmHg, and estimated glomerular filtration rate ≥ 30 mL/min/1.73 m(2) to serelaxin (n = 34) or placebo (n = 37) within 48 h of hospitalization. Co-primary endpoints were peak change from baseline in PCWP and cardiac index (CI) during the first 8 h of infusion. Among 63 patients eligible for haemodynamic analysis (serelaxin, n = 32; placebo, n = 31), those treated with serelaxin had a significantly higher decrease in peak PCWP during the first 8 h of infusion (difference vs. placebo: -2.44 mmHg, P = 0.004). Serelaxin showed no significant effect on the peak change in CI vs. placebo. Among secondary haemodynamic endpoints, a highly significant reduction in pulmonary artery pressure (PAP) was observed throughout the serelaxin infusion (largest difference in mean PAP vs. placebo: -5.17 mmHg at 4 h, P < 0.0001). Right atrial pressure, systemic/pulmonary vascular resistance, and systolic/diastolic BP decreased from baseline with serelaxin vs. placebo and treatment differences reached statistical significance at some time points. Serelaxin administration improved renal function and decreased N-terminal pro-brain natriuretic peptide levels vs. placebo. Treatment with serelaxin was well tolerated with no apparent safety concerns.
Conclusion: The haemodynamic effects of serelaxin observed in the present study provide plausible mechanistic support for improvement in signs and symptoms of congestion observed with this agent in AHF patients. ClinicalTrials.gov identifier NCT01543854.
Keywords: Acute heart failure; Clinical trial; Haemodynamics; Serelaxin.
Figures

Comment in
-
Serelaxin: insights into its haemodynamic, biochemical, and clinical effects in acute heart failure.Eur Heart J. 2014 Feb;35(7):410-2. doi: 10.1093/eurheartj/eht477. Epub 2013 Nov 24. Eur Heart J. 2014. PMID: 24277752 No abstract available.
References
-
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. - PubMed
-
- Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. - PubMed
-
- Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, Baumann G, Stangl K. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail. 2009;15:182–190. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

