Anti-transcription intermediary factor 1-gamma autoantibody ELISA development and validation
- PMID: 24255164
- PMCID: PMC3930887
- DOI: 10.1093/rheumatology/ket383
Anti-transcription intermediary factor 1-gamma autoantibody ELISA development and validation
Abstract
Objectives: A quantitative anti-transcription intermediary factor 1-gamma (anti-TIF1-γ) ELISA may improve the detection of cancer-associated myositis (CAM). The aims of this study were the development and validation of a quantitative anti-TIF1-γ autoantibody ELISA in patients with myositis.
Methods: We developed an ELISA using recombinant purified full-length human TIF1-γ. Patient serum was incubated with TIF1-γ-coated ELISA plates, and secondary antibody that bound human IgG was used to detect anti-TIF1-γ binding. Protein immunoprecipitation (IP) was used as the gold standard for the presence of anti-TIF1-γ. Serum samples from myositis patients with positive and negative anti-TIF1-γ by IP, from non-myositis autoimmune patients (SSc, SLE and RA) and from healthy controls were analysed. The ELISA's sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were evaluated. Agreement between the ELISA and IP results was determined using chi-squared and kappa tests. Test-retest reliability of the ELISA was assessed.
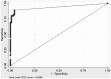
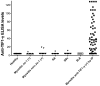
Results: We identified 55 myositis patients with and 111 controls without anti-TIF1-γ by IP. Anti-TIF1-γ positivity by ELISA showed strong agreement (93.9%) with IP results (κ = 0.87). The sensitivity, specificity, PPV, NPV and overall accuracy of the anti-TIF1-γ ELISA were 91%, 96%, 93%, 95% and 94%, respectively. The area under the curve (AUC) of a receiver operating characteristic (ROC) curve was 0.938. Test-retest reliability was strong (Pearson r = 0.913, P < 0.001).
Conclusion: We developed a quantitative ELISA for detecting serum anti-TIF1-γ autoantibodies and validated the assay in myositis and other connective tissue disease patients. The availability of a validated, quantitative ELISA should improve the detection of anti-TIF1-γ autoantibodies and may improve the detection of CAM.
Keywords: ELISA; anti-transcription intermediary factor 1-gamma (TIF1-γ) autoantibody; development and validation of quantitative measure; idiopathic inflammatory myopathy; immunoprecipitation.
Figures
References
-
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. - PubMed
-
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. - PubMed
-
- Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O’Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64:523–32. - PubMed
-
- Fujimoto M, Hamaguchi Y, Kaji K, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 2012;64:513–22. - PubMed
-
- Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, et al. Anti-TIF1gamma antibodies (anti-p155) in adult patients with dermatomyositis: comparison of different diagnostic assays. Ann Rheum Dis. 2012;71:993–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical