Cardiac regeneration: current therapies-future concepts
- PMID: 24255783
- PMCID: PMC3815722
- DOI: 10.3978/j.issn.2072-1439.2013.08.71
Cardiac regeneration: current therapies-future concepts
Abstract
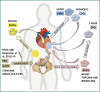
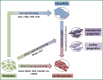
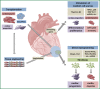
Cardiovascular disease (CVD) continues to be one of the main causes of death in the western world. A high burden of disease and the high costs for the healthcare systems claim for novel therapeutic strategies besides current conventional medical care. One decade ago first clinical trials addressed stem cell based therapies as a potential alternative therapeutic strategy for myocardial regeneration and repair. Besides bone marrow derived stem cells (BMCs), adult stem cells from adipose or cardiac tissue have been used in current clinical studies with inconsistent results. Although outcomes in terms of safety and feasibility are generally encouraging, functional improvements were mostly disappointingly low and have failed to reach expectations. In the future, new concepts for myocardial regeneration, especially concerning recovery of cardiomyocyte loss, have to be developed. Transplantation of novel stem or progenitor cell populations with "true" regenerative potential, direct reprogramming of scar tissue into functional myocardium, tissue engineering or stimulation of endogenous cardiac repair by pharmacological agents are conceivable. This review summarizes current evidence of stem cell based regenerative therapies and discusses future strategies to improve functional outcomes.
Keywords: Myocardial infarction; regenerative medicine; reprogramming; stem cells; tissue engineering.
Figures
References
-
- Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 2013;61:2329-38 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
