Searching for nuclear export elements in hepatitis D virus RNA
- PMID: 24255883
- PMCID: PMC3832856
- DOI: 10.5501/wjv.v2.i3.123
Searching for nuclear export elements in hepatitis D virus RNA
Abstract
Aim: To search for the presence of cis elements in hepatitis D virus (HDV) genomic and antigenomic RNA capable of promoting nuclear export.
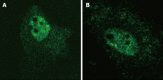
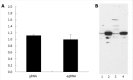
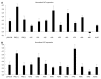
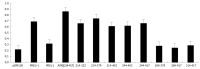
Methods: We made use of a well characterized chloramphenicol acetyl-transferase reporter system based on plasmid pDM138. Twenty cDNA fragments corresponding to different HDV genomic and antigenomic RNA sequences were inserted in plasmid pDM138, and used in transfection experiments in Huh7 cells. The relative amounts of HDV RNA in nuclear and cytoplasmic fractions were then determined by real-time polymerase chain reaction and Northern blotting. The secondary structure of the RNA sequences that displayed nuclear export ability was further predicted using a web interface. Finally, the sensitivity to leptomycin B was assessed in order to investigate possible cellular pathways involved in HDV RNA nuclear export.
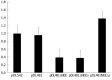
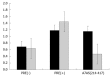
Results: Analysis of genomic RNA sequences did not allow identifying an unequivocal nuclear export element. However, two regions were found to promote the export of reporter mRNAs with efficiency higher than the negative controls albeit lower than the positive control. These regions correspond to nucleotides 266-489 and 584-920, respectively. In addition, when analyzing antigenomic RNA sequences a nuclear export element was found in positions 214-417. Export mediated by the nuclear export element of HDV antigenomic RNA is sensitive to leptomycin B suggesting a possible role of CRM1 in this transport pathway.
Conclusion: A cis-acting nuclear export element is present in nucleotides 214-417 of HDV antigenomic RNA.
Keywords: Antigenomic RNA; Genomic RNA; Hepatitis D virus; Nuclear export; Nuclear export element.
Figures

References
-
- Smedile A, Rizzetto M. HDV: thirty years later. Dig Liver Dis. 2011;43 Suppl 1:S15–S18. - PubMed
-
- Govindarajan S, Chin KP, Redeker AG, Peters RL. Fulminant B viral hepatitis: role of delta agent. Gastroenterology. 1984;86:1417–1420. - PubMed
-
- Jacobson IM, Dienstag JL, Werner BG, Brettler DB, Levine PH, Mushahwar IK. Epidemiology and clinical impact of hepatitis D virus (delta) infection. Hepatology. 1985;5:188–191. - PubMed
-
- Smedile A, Rizzetto M, Gerin JL. Advances in hepatitis D virus biology and disease. Prog Liver Dis. 1994;12:157–175. - PubMed
-
- Taylor JM. Replication of human hepatitis delta virus: recent developments. Trends Microbiol. 2003;11:185–190. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

