CD169 mediates the capture of exosomes in spleen and lymph node
- PMID: 24255917
- PMCID: PMC3888287
- DOI: 10.1182/blood-2013-03-489732
CD169 mediates the capture of exosomes in spleen and lymph node
Abstract
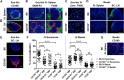
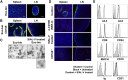
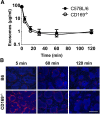
Exosomes are lipid nanovesicles released following fusion of the endosoma limiting membrane with the plasma membrane; however, their fate in lymphoid organs after their release remains controversial. We determined that sialoadhesin (CD169; Siglec-1) is required for the capture of B cell-derived exosomes via their surface-expressed α2,3-linked sialic acids. Exosome-capturing macrophages were present in the marginal zone of the spleen and in the subcapsular sinus of the lymph node. In vitro assays performed on spleen and lymph node sections confirmed that exosome binding to CD169 was not solely due to preferential fluid flow to these areas. Although the circulation half-life of exosomes in blood of wild-type and CD169(-/-) mice was similar, exosomes displayed altered distribution in CD169(-/-) mice, with exosomes freely accessing the outer marginal zone rim of SIGN-R1(+) macrophages and F4/80(+) red pulp macrophages. In the lymph node, exosomes were not retained in the subcapsular sinus of CD169(-/-) mice but penetrated deeper into the paracortex. Interestingly, CD169(-/-) mice demonstrated an enhanced response to antigen-pulsed exosomes. This is the first report of a role for CD169 in the capture of exosomes and its potential to mediate the immune response to exosomal antigen.
Figures




References
-
- Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. - PubMed
-
- André F, Chaput N, Schartz NE, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–2136. - PubMed
-
- Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–1981. - PubMed
-
- Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267(2):583-590. - PubMed
-
- Wubbolts R, Leckie RS, Veenhuizen PT, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–10972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

