Trichomonas vaginalis flavin reductase 1 and its role in metronidazole resistance
- PMID: 24256032
- PMCID: PMC4437529
- DOI: 10.1111/mmi.12455
Trichomonas vaginalis flavin reductase 1 and its role in metronidazole resistance
Abstract
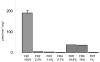
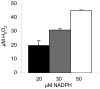
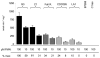
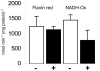
The enzyme flavin reductase 1 (FR1) from Trichomonas vaginalis, formerly known as NADPH oxidase, was isolated and identified. Flavin reductase is part of the antioxidative defence in T. vaginalis and indirectly reduces molecular oxygen to hydrogen peroxide via free flavins. Importantly, a reduced or absent flavin reductase activity has been reported in metronidazole-resistant T. vaginalis, resulting in elevated intracellular oxygen levels and futile cycling of metronidazole. Interestingly, FR1 has no close homologue in any other sequenced genome, but seven full-length and three truncated isoforms exist in the T. vaginalis genome. However, out of these, only FR1 has an affinity for flavins, i.e. FMN, FAD and riboflavin, which is high enough to be of physiological relevance. Although there are no relevant changes in the gene sequence or any alterations of the predicted FR1-mRNA structure in any of the strains studied, FR1 is not expressed in highly metronidazole-resistant strains. Transfection of a metronidazole-resistant clinical isolate (B7268), which does not express any detectable amounts of FR, with a plasmid bearing a functional FR1 gene nearly completely restored metronidazole sensitivity. Our results indicate that FR1 has a significant role in the emergence of metronidazole resistance in T. vaginalis.
© 2013 John Wiley & Sons Ltd.
Figures





Similar articles
-
The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways.Mol Biochem Parasitol. 2010 May;171(1):17-24. doi: 10.1016/j.molbiopara.2010.01.001. Epub 2010 Jan 20. Mol Biochem Parasitol. 2010. PMID: 20093143
-
Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance.Mol Microbiol. 2009 Apr;72(2):518-36. doi: 10.1111/j.1365-2958.2009.06675.x. Mol Microbiol. 2009. PMID: 19415801
-
Down-regulation of flavin reductase and alcohol dehydrogenase-1 (ADH1) in metronidazole-resistant isolates of Trichomonas vaginalis.Mol Biochem Parasitol. 2012 Jun;183(2):177-83. doi: 10.1016/j.molbiopara.2012.03.003. Epub 2012 Mar 17. Mol Biochem Parasitol. 2012. PMID: 22449940 Free PMC article.
-
A systematic review of the literature on mechanisms of 5-nitroimidazole resistance in Trichomonas vaginalis.Parasitology. 2020 Nov;147(13):1383-1391. doi: 10.1017/S0031182020001237. Epub 2020 Jul 30. Parasitology. 2020. PMID: 32729451 Free PMC article.
-
Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis.Clin Microbiol Rev. 2004 Oct;17(4):783-93, table of contents. doi: 10.1128/CMR.17.4.783-793.2004. Clin Microbiol Rev. 2004. PMID: 15489348 Free PMC article. Review.
Cited by
-
Culturing of Giardia lamblia under microaerobic conditions can impact metronidazole susceptibility by inducing increased expression of antioxidant enzymes.Int J Parasitol Drugs Drug Resist. 2025 Apr;27:100585. doi: 10.1016/j.ijpddr.2025.100585. Epub 2025 Feb 1. Int J Parasitol Drugs Drug Resist. 2025. PMID: 39904006 Free PMC article.
-
Recent advances in the molecular biology of the protist parasite Trichomonas vaginalis.Fac Rev. 2021 Mar 4;10:26. doi: 10.12703/r/10-26. eCollection 2021. Fac Rev. 2021. PMID: 33718943 Free PMC article. Review.
-
Characterization of Metronidazole-Resistant Giardia intestinalis Lines by Comparative Transcriptomics and Proteomics.Front Microbiol. 2022 Feb 10;13:834008. doi: 10.3389/fmicb.2022.834008. eCollection 2022. Front Microbiol. 2022. PMID: 35222342 Free PMC article.
-
A Comparison of Bottom-Up Proteomic Sample Preparation Methods for the Human Parasite Trichomonas vaginalis.ACS Omega. 2024 Feb 13;9(8):9782-9791. doi: 10.1021/acsomega.3c10040. eCollection 2024 Feb 27. ACS Omega. 2024. PMID: 38434803 Free PMC article.
-
Genetic Indicators of Drug Resistance in the Highly Repetitive Genome of Trichomonas vaginalis.Genome Biol Evol. 2017 Jun 1;9(6):1658-1672. doi: 10.1093/gbe/evx110. Genome Biol Evol. 2017. PMID: 28633446 Free PMC article.
References
-
- Chapman A, Cammack R, Linstead R, Lloyd D. The generation of metronidazole radicals in hydrogenosomes isolated from Trichomonas vaginalis. J Gen Microbiol. 1985;131:2141–2144. - PubMed
-
- Coombs GH, Westrop GD, Suchan P, Puzova G, Hirt RP, Embley TM, et al. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J Biol Chem. 2004;279:5249–5256. - PubMed
-
- Cui J, Smith TF, Samuelson J. Gene expansion in Trichomonas vaginalis: a case study on transmembrane cyclases. Genome Inform. 2007;18:35–43. - PubMed
-
- Davis SR, Lushbaugh WB. Oxidative stress and Trichomonas vaginalis: the effect of hydrogen peroxide in vitro. Am J Trop Med Hyg. 1993;48:480–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

