Cooperative self-assembly of peptide gelators and proteins
- PMID: 24256076
- PMCID: PMC4374667
- DOI: 10.1021/bm401319c
Cooperative self-assembly of peptide gelators and proteins
Abstract
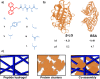
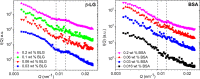
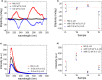
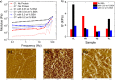
Molecular self-assembly provides a versatile route for the production of nanoscale materials for medical and technological applications. Herein, we demonstrate that the cooperative self-assembly of amphiphilic small molecules and proteins can have drastic effects on supramolecular nanostructuring of resulting materials. We report that mesoscale, fractal-like clusters of proteins form at concentrations that are orders of magnitude lower compared to those usually associated with molecular crowding at room temperature. These protein clusters have pronounced effects on the molecular self-assembly of aromatic peptide amphiphiles (fluorenylmethoxycarbonyl- dipeptides), resulting in a reversal of chiral organization and enhanced order through templating and binding. Moreover, the morphological and mechanical properties of the resultant nanostructured gels can be controlled by the cooperative self-assembly of peptides and protein fractal clusters, having implications for biomedical applications where proteins and peptides are both present. In addition, fundamental insights into cooperative interplay of molecular interactions and confinement by clusters of chiral macromolecules is relevant to gaining understanding of the molecular mechanisms of relevance to the origin of life and development of synthetic mimics of living systems.
Figures



References
-
- Lehn J. M.; Supramolecular Chemistry – concepts and perspectives: VCH: Weinheim, Germany, 1995;
- Whitesides G. M.; Grzybowski B. Science 2002, 295, 2418–21. - PubMed
- Aida T.; Meijer E. W.; Stupp S. I. Science 2012, 335, 813–817. - PMC - PubMed
- Fichman G.; Gazit E. Acta Biomater. 2013, DOI: 10.1016/j.bbr.2011.03.031. - PubMed
-
- Yang Z.; Gu H.; Fu D.; Gao P.; Lam J. K.; Xu B. Adv. Mater. 2004, 16, 1440–1444.
- Hirst A. R.; Roy S.; Arora M.; Das A. K.; Hodson N.; Murray P.; Marshall S.; Javid N.; Sefcik J.; Boekhoven J.; van Esch J. H.; Santabarbara S.; Hunt N. T.; Ulijn R. V. Nat. Chem. 2010, 2, 1089–1094. - PubMed
- Gao Y.; Shi J.; Yuan D.; Xu B. Nat. Commun. 2012, 3, 1033. - PMC - PubMed
- Hirst A. R.; Escuder B.; Miravet J. F.; Smith D. K. Angew. Chem., Int. Ed. 2008, 47, 8002–8020. - PubMed
- Fleming S.; Debnath S.; Frederix P. W. J. M.; Tuttle T.; Ulijn R. V. Chem. Commun. 2013, 49, 10587–10589. - PubMed
-
- Ellis R. J.; Minton A. P. Nature 2003, 425, 27–28. - PubMed
-
- Minton A. P. Curr. Opi. in Str. Biol. 2000, 10, 34–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

