Nickel oral hyposensitization in patients with systemic nickel allergy syndrome
- PMID: 24256166
- PMCID: PMC4673509
- DOI: 10.3109/07853890.2013.861158
Nickel oral hyposensitization in patients with systemic nickel allergy syndrome
Abstract
Background: This is the first randomized, double-blind, placebo-controlled trial (EUDRACT No. 2009-013923-43) evaluating nickel oral hyposensitizing treatment (NiOHT) in patients with "systemic nickel allergy syndrome" (SNAS), characterized by Ni-allergic contact dermatitis and systemic reactions after eating Ni-rich food.
Methods: Adults with positive Ni-patch test, who reported symptoms suggesting SNAS, which improved after Ni-poor diet, and were positive to Ni-oral challenge were eligible. Patients were randomly assigned to three treatments (1.5 μg, 0.3 μg, or 30 ng Ni/week) or placebo for a year, with progressive reintroduction of Ni-rich foods form the 5(th) month. Out of 141 patients randomized, 113 completed the trial. Endpoints were efficacy and tolerability of treatment.
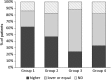
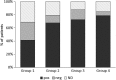
Results: During Ni-rich food re-introduction, the 1.5 μg Ni/week group had a mean VAS score significantly higher than placebo (p = 0.044), with significant improvement of gastrointestinal symptoms (p = 0.016;) and significantly fewer rescue medications. Cutaneous manifestations also improved but without reaching statistical significance. After the treatment, oral challenge with higher Ni doses than at baseline were needed to cause symptoms to flare-up in significantly more patients given 1.5 μg Ni/week than placebo (p = 0.05). Patients reported no side-effects.
Conclusions: NiOHT is effective in SNAS, in particular on gastrointestinal manifestations, with trend toward improvement of cutaneous symptoms.
Figures


References
-
- Thyssen JP, Linneberg A, Menné T, Johansen JD. The epidemiology of contact allergy in the general population – prevalence and main findings. Contact Dermatitis. 2007;57:287–99. - PubMed
-
- Jacob SE, Moennich JN, McKean BA. Nickel allergy in the United States: A public health issue in need of a “nickel directive”. J Am Acad Dermatol. 2009;60:1067–9. - PubMed
-
- Schafer T, Bohler E, Ruhdorfer S, Weigl L, Wessner D, Ilipiak B. Epidemiology of contact allergy in adults. Allergy. 2001;56:1192–6. - PubMed
-
- Jensen CS, Menné T, Johansen JD. Systemic contact dermatitis after oral exposure to nickel: a review with a modified meta-analysis. Contact Dermatitis. 2006;54:79–86. - PubMed
-
- Braga M, Quecchia C, Perotta C, Timpini A, Maccarinelli K, Di Tommaso L. Systemic nickel allergy syndrome: nosologic framework and diet regimen. Int J Immunopthol Pharmacol. 2013;26:707–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
