Recent advances in the discovery of small molecules targeting exchange proteins directly activated by cAMP (EPAC)
- PMID: 24256330
- PMCID: PMC4016168
- DOI: 10.1021/jm401425e
Recent advances in the discovery of small molecules targeting exchange proteins directly activated by cAMP (EPAC)
Abstract
3',5'-Cyclic adenosine monophosphate (cAMP) is a pivotal second messenger that regulates numerous biological processes under physiological and pathological conditions, including cancer, diabetes, heart failure, inflammation, and neurological disorders. In the past, all effects of cAMP were initially believed to be mediated by protein kinase A (PKA) and cyclic nucleotide-regulated ion channels. Since the discovery of exchange proteins directly activated by cyclic adenosine 5'-monophosphate (EPACs) in 1998, accumulating evidence has demonstrated that the net cellular effects of cAMP are also regulated by EPAC. The pursuit of the biological functions of EPAC has benefited from the development and applications of a growing number of pharmacological probes targeting EPACs. In this review, we seek to provide a concise update on recent advances in the development of chemical entities including various membrane-permeable analogues of cAMP and newly discovered EPAC-specific ligands from high throughput assays and hit-to-lead optimizations.
Conflict of interest statement
CONFLICT OF INTERESTS
The authors declare no competing financial interest
Figures

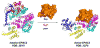

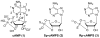
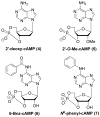
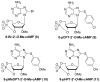

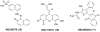
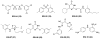
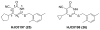
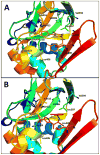
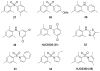
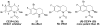
References
-
- Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. - PubMed
-
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. - PubMed
-
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. - PubMed
-
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. - PubMed
-
- Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;186:167–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

