The PanAM study: a multi-center, double-blinded, randomized, non-inferiority study of paracetamol versus non-steroidal anti-inflammatory drugs in treating acute musculoskeletal trauma
- PMID: 24256450
- PMCID: PMC4225513
- DOI: 10.1186/1471-227X-13-19
The PanAM study: a multi-center, double-blinded, randomized, non-inferiority study of paracetamol versus non-steroidal anti-inflammatory drugs in treating acute musculoskeletal trauma
Abstract
Background: Acute musculoskeletal trauma, including strains, sprains or contusions, occur frequently. Pain management is a crucial component of treatment. However, there is no convincing evidence which drug is superior in managing pain in these patients. The aim of the PanAM Study is to compare analgesic efficacy of three strategies of pain management: paracetamol, diclofenac, or a combination of both in patients with acute musculoskeletal trauma.
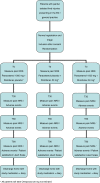
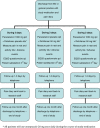
Methods/design: The PanAM Study is a multi-center, double blind randomized controlled trial with non-inferiority design. Included are adult patients presenting to an academic, urban Emergency Department or to a General Practice with acute, blunt, traumatic limb injury. In total, 547 patients will be included using a predefined list of exclusion criteria, to be allocated by randomization to treatment with paracetamol + placebo diclofenac, diclofenac + placebo paracetamol or paracetamol + diclofenac. The hypothesis is that paracetamol will not be inferior to treatment with diclofenac, or the combination of both. Primary outcome will be between-group differences in decrease in pain, measured with Numerical Rating Scales at baseline and at 90 minutes after study drug administration. Secondary outcomes are Numerical Rating Scales at 30 and 60 minutes and measured frequently during three consecutive days after discharge; occurrence of adverse effects; patient satisfaction and an analysis of quality of life and cost-effectiveness. Recruitment started July 2013 and is expected to last a year.
Discussion: With this multi-center randomized clinical trial we will investigate whether treatment with paracetamol alone is not inferior to diclofenac alone or a combination of both drugs in adult patients with acute musculoskeletal trauma. The main relevance of the trial is to demonstrate the benefits and risks of three commonly used treatment regimens for musculoskeletal trauma. Data that lead to the prevention of severe Non-Steroidal Anti-Inflammatory Drugs-related adverse effects might be gathered.
Trial registration: Dutch Trial Register (http://www.trialregister.nl): NTR3982.EudraCT database (http://www.clinicaltrialsregister.eu): 201300038111.
Figures
References
-
- Den Hertog P, Stam C, Valkenberg H, Bloemhoff A, Panneman M, Klein Wolt K. Letsels en letselpreventie. Amsterdam: VeiligheidNL; 2013. Letsel in Nederland; p. 14. In Dutch.
-
- United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States. 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
-
- Gøtsche PC. NSAIDs. Clin Evid. 2010;06:1108.
-
- Harvey R. Musculoskeletal disorders: Managing sprains and strains. Pharma J. 1997;259:292–295.
-
- McGriff-Lee N. Management of acute soft tissue injuries. J Pharm Pract. 2003;16:51–58. doi: 10.1177/0897190002239634. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

