Nicorandil inhibits inflammasome activation and Toll-like receptor-4 signal transduction to protect against oxygen-glucose deprivation-induced inflammation in BV-2 cells
- PMID: 24256503
- PMCID: PMC6493004
- DOI: 10.1111/cns.12178
Nicorandil inhibits inflammasome activation and Toll-like receptor-4 signal transduction to protect against oxygen-glucose deprivation-induced inflammation in BV-2 cells
Abstract
Background and purpose: Our previous studies have demonstrated adenosine triphosphate-sensitive potassium channel (KATP channel) openers could protect against inflammatory response in brain disease, but little is known about the mechanisms involved in KATP channel openers inhibiting neuroinflammation.
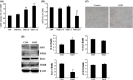
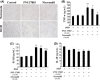
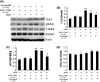
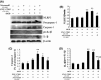
Methods and results: In the present study, we found that oxygen-glucose deprivation (OGD) resulted in BV-2 cells activation, significantly increased tumor necrosis factor-alpha and interleukin-1beta (IL-1β) levels, accompanied by downregulating Kir6.1 subunit. Pretreatment with nicorandil, a KATP channel opener, could attenuate OGD-induced BV-2 cells activation and inhibit pro-inflammatory factors release. Further study demonstrated that OGD activated Toll-like receptor-4 (TLR4) signaling pathway and NOD-like receptor pyrin domain containing three inflammasome, thereby increased IL-1β production. Pretreatment with nicorandil could reverse the two pathways involved in IL-1β production.
Conclusions: Our findings reveal that KATP channel openers could protect against OGD-induced neuroinflammation via inhibiting inflammasome activation and TLR4 signal transduction.
Keywords: ATP-sensitive potassium channel; Inflammasome; Neuroinflammation; Nicorandil; Oxygen-glucose deprivation.
© 2013 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

