Heat shock factor 1 over-expression protects against exposure of hydrophobic residues on mutant SOD1 and early mortality in a mouse model of amyotrophic lateral sclerosis
- PMID: 24256636
- PMCID: PMC3907013
- DOI: 10.1186/1750-1326-8-43
Heat shock factor 1 over-expression protects against exposure of hydrophobic residues on mutant SOD1 and early mortality in a mouse model of amyotrophic lateral sclerosis
Abstract
Background: Mutations in the Cu/Zn superoxide dismutase gene (SOD1) are responsible for 20% of familial forms of amyotrophic lateral sclerosis (ALS), and mutant SOD1 has been shown to have increased surface hydrophobicity in vitro. Mutant SOD1 may adopt a complex array of conformations with varying toxicity in vivo. We have used a novel fluorescence-based proteomic assay using 4,4'-bis-1-anilinonaphthalene-8-sulfonate (bisANS) to assess the surface hydrophobicity, and thereby distinguish between different conformations, of SOD1 and other proteins in situ.
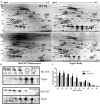
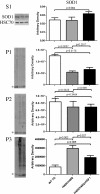
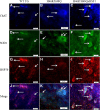
Results: Covalent bisANS labeling of spinal cord extracts revealed that alterations in surface hydrophobicity of H46R/H48Q mutations in SOD1 provoke formation of high molecular weight SOD1 species with lowered solubility, likely due to increased exposure of hydrophobic surfaces. BisANS was docked on the H46R/H48Q SOD1 structure at the disordered copper binding and electrostatic loops of mutant SOD1, but not non-mutant WT SOD1. 16 non-SOD1 proteins were also identified that exhibited altered surface hydrophobicity in the H46R/H48Q mutant mouse model of ALS, including proteins involved in energy metabolism, cytoskeleton, signaling, and protein quality control. Heat shock proteins (HSPs) were also enriched in the detergent-insoluble fractions with SOD1. Given that chaperones recognize proteins with exposed hydrophobic surfaces as substrates and the importance of protein homeostasis in ALS, we crossed SOD1 H46R/H48Q mutant mice with mice over-expressing the heat shock factor 1 (HSF1) transcription factor. Here we showed that HSF1 over-expression in H46R/H48Q ALS mice enhanced proteostasis as evidenced by increased expression of HSPs in motor neurons and astrocytes and increased solubility of mutant SOD1. HSF1 over-expression significantly reduced body weight loss, delayed ALS disease onset, decreases cases of early disease, and increased survival for the 25th percentile in an H46R/H48Q SOD1 background. HSF1 overexpression did not affect macroautophagy in the ALS background, but was associated with maintenance of carboxyl terminus of Hsp70 interacting protein (CHIP) expression which declined in H46R/H48Q mice.
Conclusion: Our results uncover the potential importance of changes in protein surface hydrophobicity of SOD1 and other non-SOD1 proteins in ALS, and how strategies that activate HSF1 are valid therapies for ALS and other age-associated proteinopathies.
Figures



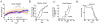

References
-
- Lelie HL, Liba A, Bourassa MW, Chattopadhyay M, Chan PK, Gralla EB, Miller LM, Borchelt DR, Valentine JS, Whitelegge JP. Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice. The Journal of biological chemistry. 2011;8:2795–2806. doi: 10.1074/jbc.M110.186999. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

