Opportunities for live cell FT-infrared imaging: macromolecule identification with 2D and 3D localization
- PMID: 24256815
- PMCID: PMC3856089
- DOI: 10.3390/ijms141122753
Opportunities for live cell FT-infrared imaging: macromolecule identification with 2D and 3D localization
Abstract
Infrared (IR) spectromicroscopy, or chemical imaging, is an evolving technique that is poised to make significant contributions in the fields of biology and medicine. Recent developments in sources, detectors, measurement techniques and speciman holders have now made diffraction-limited Fourier transform infrared (FTIR) imaging of cellular chemistry in living cells a reality. The availability of bright, broadband IR sources and large area, pixelated detectors facilitate live cell imaging, which requires rapid measurements using non-destructive probes. In this work, we review advances in the field of FTIR spectromicroscopy that have contributed to live-cell two and three-dimensional IR imaging, and discuss several key examples that highlight the utility of this technique for studying the structure and chemistry of living cells.
Figures
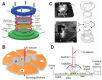


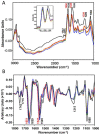
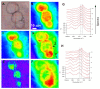

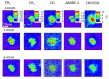
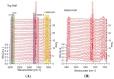
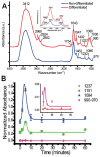


References
-
- Martin M.C., Schade U., Lerch P., Dumas P. Recent applications and current trends in analytical chemistry using synchrotron-based Fourier-transform infrared microspectroscopy. Trends Anal. Chem. 2010;29:453–463.
-
- Hirschmugl C.J., Gough K.M. Fourier transform infrared spectrochemical imaging: Review of design and applications with a focal plane array and multiple beam synchrotron radiation source. Appl. Spectrosc. 2012;66:475–491. - PubMed
-
- Nasse M.J., Ratti S., Giordano M., Hirschmugl C.J. Demountable liquid/flow cell for in vivo infrared microspectroscopy of biological specimens. Appl. Spectrosc. 2009;63:1181–1186. - PubMed
-
- Tobin M.J., Puskar L., Hasan J., Webb H.K., Hirschmugl C.J., Nasse M.J., Gervinskas G., Juodkazis S., Watson G.S., Watson J.A., et al. High-spatial-resolution mapping of superhydro-phobic cicada wing surface chemistry using infrared microspectroscopy and infrared imaging at two synchrotron beamlines. J. Synchrot. Radiat. 2013;20:482–489. - PubMed
-
- Holman H.Y.N., Miles R., Hao Z., Wozei E., Anderson L.M., Yang H. Real-time chemical imaging of bacterial activity in biofilms using open-channel microfluidics and synchrotron FTIR spectromicroscopy. Anal. Chem. 2009;81:8564–8570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

