In-ice evolution of RNA polymerase ribozyme activity
- PMID: 24256864
- PMCID: PMC3920166
- DOI: 10.1038/nchem.1781
In-ice evolution of RNA polymerase ribozyme activity
Abstract
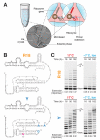
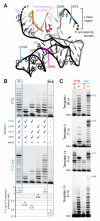
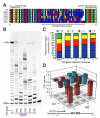
Mechanisms of molecular self-replication have the potential to shed light on the origins of life. In particular, self-replication through RNA-catalysed templated RNA synthesis is thought to have supported a primordial 'RNA world'. However, existing polymerase ribozymes lack the capacity to synthesize RNAs approaching their own size. Here, we report the in vitro evolution of such catalysts directly in the RNA-stabilizing medium of water ice, which yielded RNA polymerase ribozymes specifically adapted to sub-zero temperatures and able to synthesize RNA in ices at temperatures as low as -19 °C. The combination of cold-adaptive mutations with a previously described 5' extension operating at ambient temperatures enabled the design of a first polymerase ribozyme capable of catalysing the accurate synthesis of an RNA sequence longer than itself (adding up to 206 nucleotides), an important stepping stone towards RNA self-replication.
Figures


Comment in
-
Origin of life: Cold-hearted RNA heats up life.Nat Chem. 2013 Dec;5(12):987-9. doi: 10.1038/nchem.1811. Nat Chem. 2013. PMID: 24256858
References
-
- RNA Worlds . In: Atkins JF, Gesteland RF, Cech TR, editors. Cold Spring Harbor Laboratory Press; 2011.
-
- Gilbert W. Origin of life: The RNA world. Nature. 1986;319:618–618.
-
- Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

